You are here
Epigenetics in the genes

“I was not the kind of child to capture insects and observe them in a jar, but I was curious about everything, and had a thirst to get to the bottom of things.” Edith Heard, who was born in the United Kingdom in 1965 to an English father and a Greek mother, was fascinated from a very young age by the sciences, mathematics in particular. Her brilliant studies led her to the prestigious University of Cambridge, where she initially planned to become an astrophysicist. “That seemed natural at the time, as I was good at maths and physics, and very keen on astronomy.”
But she soon traded stars for biology, a move that she believes bears her mother’s influence. “She regularly hosted people from Greece travelling to London for treatment. So I grew up in a world of combatting illnesses, and in a home that was always open to others.” This must be the reason for the generosity we see in her, in her light blue gaze and kind smiles. “When I developed a passion for biology at university, I wanted to help treat people, and even thought of becoming a doctor. But my craving to understand things won out. I told myself that decoding the living world would better help to combat diseases.”
Post-it notes on DNA molecules
She began this effort with a doctoral thesis in biochemistry at the Imperial Cancer Research Fund in London, where she, somewhat by chance, came face-to-face with epigenetics. To characterise the genome of a cancer cell line, she needed to cut DNA with what are known as restriction enzymes from bacteria. The only problem is that these enzymes do not function when the DNA strands to be cut are subject to methylation, a chemical change that is none other than an epigenetic mark. While gathering material on how to eliminate this methylation, she discovered a vibrant and fascinating area of research, which was growing rapidly at the time.

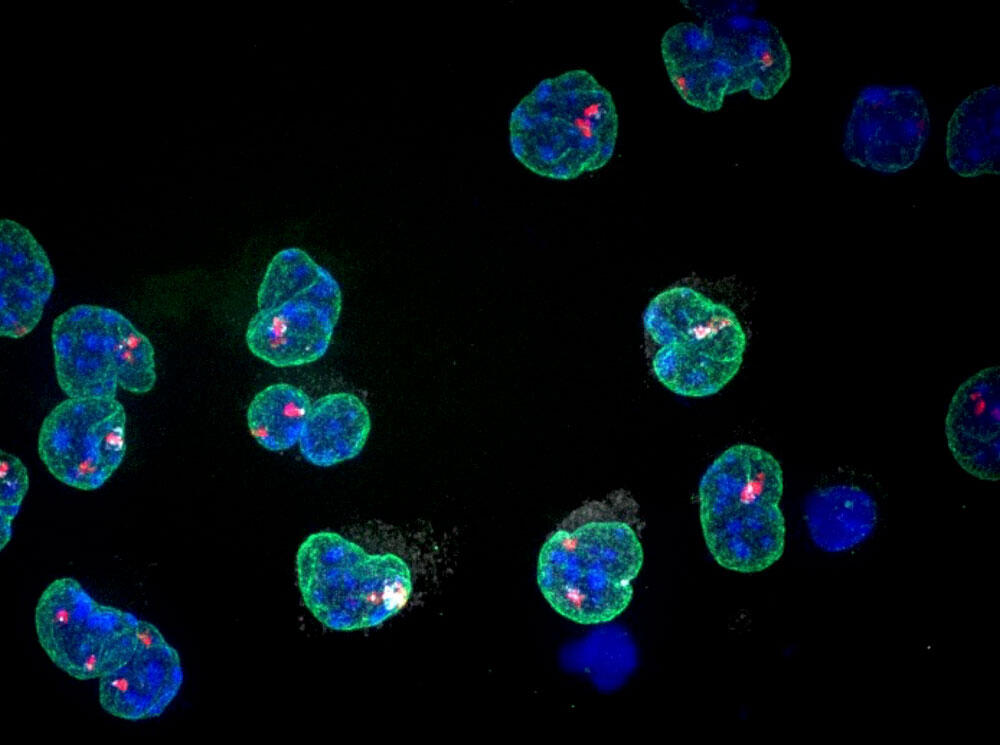
Rather like post-it notes placed on DNA molecules, epigenetic marks take part in the expression – or conversely the repression – of genes via chemical modifications, such as methylation. Unlike genetic mutations however, they do not alter the DNA sequence, and are reversible.
The epigenome – the layer of information that complements the genome – is indispensable to cellular differentiation, and plays a key role in embryogenesis. Since each cell carries the entire DNA code, it is these epigenetic marks that enable them to become specialised, turning into a liver cell, heart cell, or muscle cell. In particular, epigenetic marks also allow cells to memorise their state, and transmit it to ensuing generations of cells. “Epigenetics is therefore a cellular memory of sorts,” Heard summarises.
While it has not been proved that epigenetic modifications are transmitted from parents to children in mammals – “Suppose individuals had all of the epigenetic marks resulting from the events experienced by their forebears, that would be a disaster for the functioning of the organism!” – some marks can nevertheless be induced by environmental factors (stress, tobacco, pollution), and are harmful to human health, without for all that being transmitted to their descendants.
In 1990, having completed her thesis, Heard embarked on a postdoctoral fellowship at the Institut Pasteur (2) on a key subject in epigenetics, X-chromosome inactivation. “In mammals, male cells have an X chromosome and a Y chromosome, but females have two X chromosomes. This double dose is highly problematic. In humans, the only viable chromosome ‘overdose’ is trisomy 21, where the chromosome in position 21 comes in three copies instead of two. Yet this chromosome is small, while its X counterpart is particularly large, with more than 1,000 genes! If both X chromosomes are expressed in a female mammal, the embryo quickly dies. This is why evolution found an ingenious way to silence one of the two.” This is made possible by these famous epigenetic post-it notes.
US experience
Eager as usual to get down to the nitty gritty, the young researcher sought to shed light on the mysteries surrounding X-chromosome inactivation. She asked herself a series of questions: how does a cell ‘know’ that it is female? How does it choose the X chromosome to inactivate and mark with an epigenetic sticky note? And what specific mechanisms bring about this silencing? Recruited by the CNRS after her postdoctoral fellowship, she endeavoured to grasp how the X-inactivation centre functions in order to answer the many questions gnawing away at her. This ambitious goal prompted her to train in new, innovative technologies.

In 2000, she left for Cold Spring Harbor Laboratory in Long Island, New York with her partner Vincent Colot – a research professor at the Paris-based École Normale Supérieure, and a plant epigeneticist – along with their two children. “It is with him, who has since become my husband, that I built my career and shared the joys and secrets of epigenetics.” On the other side of the Atlantic, using cutting-edge microscopy, she learned how to visualise the very first stages of this inactivation process inside cells.
Armed with her curiosity and the new technical skills acquired in America upon her return to France in 2001, she joined the Institut Curie, where she served for 17 years, starting as a junior team leader until she eventually became director of the Genetics and Developmental Biology Department. “That was the most interesting period of my scientific life,” she confides, in a perfect French tinged with a melodious British accent. “I conducted basic research, which sustained my passion for understanding. At the same time, my team collaborated closely with the marvellous colleagues and doctors from the Institut Curie hospital, with a view to better grasping the epigenetic changes – especially those connected to the X chromosome – potentially linked to the development of various cancers in humans.”
She even contributed to thinking on therapeutic strategies via “epi-drugs,” which are based on the reversibility of epigenetic mechanisms, and seek to eliminate abnormal marks. “For all that, I do not believe in research that is exclusively devoted to medical applications. I am a fervent defender of basic research, which enables leaps forward in knowledge that then give rise to applications.”
When nature does DIY
She herself has spurred more than just one such leap forward. In 2004, her team was one of the first to recreate the stages of X-chromosome inactivation in mice. With her collaborators, chief among them the postdoctoral researcher Ikuhiro Okamoto, whom she considers to be “extraordinarily talented”, she showed that at an embryo’s 4-cell stage, the paternal X chromosome is systematically inactivated, before being reactivated at the 120-cell stage, after which inactivation occurs randomly on one of the two X chromosomes (paternal or maternal).1
“The epigenetic dynamic that we brought to light was unexpected.” It consequently caused a stir in the scientific community, as did the next breakthrough a few years later. With Dr. Okamoto and other colleagues, she closely observed the same process in humans and rabbits. Surprisingly, the dynamic for these two species was very different to the one observed in mice: the two X chromosomes are first inactivated simultaneously, then one is so to speak “reactivated”, while the other remains silenced. “We were expecting that a process so central to biology would be conserved by evolution, and would be similar from one species to another, but that’s not the case. Actually, nature makes do with what it has at hand, and adapts to the developmental needs and constraints of each species.”

These results were published in the journal Nature in 2011. At the time, Heard was already an internationally renowned researcher as well as the winner of numerous awards, including the CNRS Silver Medal in 2008, the European Research Council’s Advanced Investigator Award in 2010, and the Grand Prize of the French Foundation for Medical Research in 2011. In 2012 she was appointed as Professor at the Collège de France, and that same year published a landmark article. “With Elphège Nora, my brilliant student back then, we sought to understand how the process of inactivation begins, knowing that teams before us had already identified the key role played by a particular gene known as Xist.2 We collaborated with the Dutch scientist Job Dekker, who had developed new techniques for reconstructing the genome in 3D. We wanted to see how this specific gene was mobilised, and more specifically what part of the genome was controlling it.”
A major genetic breakthrough
In doing so, the researchers made a surprising discovery, revealing a previously unknown genome architecture. Their research showed that chromosomes are organised into territories that preferentially interact with one another; that this structuring is no doubt indispensable to the proper functioning of the genome; and that this organisation is apparently shared by all mammals. This is a perfect illustration of serendipity in science, the art of making chance discoveries while working on an entirely different subject.
Following this sensational finding, which paved the way for important research on the genome, Heard resumed her investigations into epigenetics. Once again thanks to the high-tech microscopy techniques she learned on the other side of the Atlantic, she was able to visualise the molecular choreography leading directly up to X-chromosome inactivation: “The Xist gene produces an RNA that literally covers the entire X chromosome, as if decorating it. This allows the latter to recruit epigenetic marks that will not only silence genes, but also make them memorise this silencing.”
European through and through
Even before the publication of this new discovery in Nature in 2020, Heard’s renown among biologists once again opened up new opportunities for her. In 2019 she was appointed director general of the European Molecular Biology Laboratory (EMBL), a unique intergovernmental organisation established 50 years ago to promote basic research in molecular biology, and to keep scientists on European soil. Profoundly European (in addition to being Greek-British, she has since become a little French as well), she accepted the position, and was entrusted with implementing a new and ambitious multi-year programme entitled “Molecules to Ecosystems” for the 2021-2026 period.
“We scientists have a tendency to exert as much control as possible over the environment in which we conduct our experiments, in order to prevent disturbances. But in reality, it is the exact opposite that occurs, for life is never isolated, but is constantly interacting in an ecosystem, taking part in communities, in symbiosis. The idea of this new programme is to study life within its natural context. Within this framework, we established a mission entitled Traversing European Coastlines (TREC)3, focusing on the dynamics of the marine and terrestrial ecosystems of European coasts.”
To assume her duties at the EMBL, she left Paris for Heidelberg, Germany, where the organisation is based, and is now departing for yet another destination, London, where in 2025 she will begin to serve as director of the Francis Crick Institute, a prestigious biomedical organisation, all while continuing her research and courses at the Collège de France, in addition to her commitment to helping others. Since 2017, she has been the patron for PAUSE (programme for the emergency hosting of scientists and artists in exile). Created in 2017 under the auspices of the Collège de France, the programme hosts foreign researchers who are in danger in their home country, and provides them with the means to pursue their research. “We receive requests constantly, and seek out institutes that are likely to host them,” points out Heard, her curiosity and generosity intact.
For further reading:
Edith Heard, the epigenetics revolution
Epigenetics rules the genome