You are here
Edith Heard, the Epigenetics Revolution

This is a discipline that has been booming since the early 2000s and the hopes, or even fantasies, it arouses have been the subject of extensive coverage. Epigenetics participates in regulating the expression of our genes through epigenetic marks. These chemical modifications to DNA help the cells in our body to acquire, and above all maintain, their identity during development. But they are also reversible, which opens perspectives for curing certain diseases that involve the epigenome. This January, a renowned global specialist in epigenetics, the biologist Edith Heard became director general of the prestigious European Molecular Biology Laboratory (EMBL), an intergovernmental research organisation that includes 29 countries. She told us more about her work and her new missions.
What is the origin of the term epigenetics?
Edith Heard: It was invented in 1942 by the British biologist Conrad Waddington in order to reconcile the worlds of genetics (genes were discovered at the turn of the 20th century) and embryology. Geneticists were focusing on heredity and the traits transmitted to subsequent generations, while embryologists were asking how they were put in place during development. Waddington wanted to create a new discipline that would cover embryogenesis (also called epigenesis since the 17th century) and genetics, so "epigenetics" combined these two words. This notion has evolved since then; we have discovered DNA and, with some initial disbelief, observed that all the cells in our body have the same DNA as the fertilised egg, although there are still differences between a liver cell, muscle cell or neuron, for example. Because the entire DNA code is conserved in the cells, the question posed by scientists was therefore how cells acquire their own identity, and how this is maintained during cell division. That’s how we arrived at our modern definition of epigenetics.
And what is that definition?
E. H.: Epigenetics refers to any change to gene expression that does not involve a modification of the DNA sequence, which is stable but remains reversible. We now know that cells acquire and keep their identity thanks to epigenetic marks: chemical differences in the DNA that never alter its sequence but make it possible to read certain genes and not others. So epigenetics is a sort of cell memory that is transmissible to future generations of cells. However, it is one that can be deleted, hence the term reversibility.
In the early 1980s, a scientist called Peter Jones experienced this phenomenon by chance. He was culturing mouse skin cells (fibroblasts) in a Petri dish, to which he had added a molecule, 5-azacytidine. A few days later, to his surprise, cells had appeared in the culture and looked completely different. He initially thought his sample had been contaminated by fungi, but it actually turned out that these cells were myotubes, or muscle cells. The 5-azacytidine had deleted the epigenetic marks of the embryonic cells and reprogrammed them as muscle cells!
Hence the idea sometimes put forward that epigenetics has put an end to the reign of the “all-genome”, this tenacious determinism imposed by our genetic code.
E. H.: It is an attractive theory, but partially false, because it is indeed the genetic code that decides to read certain genes (or not), thanks to proteins called transcription factors! The epigenetic machinery comes just after that: the purpose of the epigenetic marks that bind to the genes is to maintain this choice throughout cell divisions.
How did you become interested in epigenetics? You had initially trained in pure genetics.
E. H.: After my studies at Cambridge, I completed my PhD in cancer research at the Imperial Cancer Research Fund in London. I wanted to know why some parts of the genome are amplified in certain cancer cells, or in other words why there are several copies of the same genes. To look at the genome, we cut it with restriction enzymes obtained from bacteria, but this did not work when epigenetic marks were present. That’s how I became interested in epigenetics—it was for purely technical reasons! I found the article by Peter Jones and ordered the same molecule, 5-azacytidine, so as to be able to remove the epigenetic marks and cut the genome as I wished. I entered the world of chemical modifications through these manipulations.
You mentioned the specialisation of cells into skin or muscle cells, etc. Inversely, body cells can become stem cells again after the epigenetic marks have been removed, as demonstrated by Shinya Yamanaka, laureate of the Nobel Prize in Physiology or Medicine in 2012.
E. H.: That’s absolute proof that the genetic code is wholly conserved in somatic cells (editor’s note: the cells in our body). Nevertheless, this change does not occur at the click of a finger. Even using the right products, it takes three weeks to delete the epigenetic marks carried by the DNA in skin cells, and obtain induced pluripotent stem cells (iPSC) that are similar to embryonic stem cells. Indeed, the epigenetic marks tend to resist. That’s what I call the "epigenetic barrier", which prevents a change in the identity of our cells and protects the phenotype (appearance) of the multicellular beings that we are.
Epigenetics has also aroused many fantasies. The notions of reversibility and heritability, in particular, have given rise to a variety of interpretations. Epigenetic marks may be influenced by our environment, the air we breathe or the stress we experience—and transmissible to our children and grandchildren, for example. As a scientist, what is your position on this issue?
E. H.: I imagine you are referring to the epidemiological study on the consequences of the famine that affected the Netherlands during the Second World War. The children, and perhaps the grandchildren, of pregnant women who at that time could not ingest more than a few calories a day for weeks on end, may now be experiencing health problems linked to a dysfunctional metabolism. According to the study, this was due to changes that affected epigenetic modifications as a result of the famine, and these may have been transmitted to the children and then the grandchildren of these malnourished women.
As another example, some even claim that the stress suffered by Holocaust survivors may have been transmitted to subsequent generations via epigenetic marks. At present, there is no solid proof of this at the molecular biology level. And we all know that our behaviour, and the way we bond with our descendants, is a potent vector for transmission. All these fantasies surrounding epigenetics are both an incentive for scientists (because they reflect society’s interest in our work) and a drawback as they induce expectations we cannot always meet, potentially leading to frustration with our discipline. Science is still laying the molecular foundations of epigenetics, and they are 100% fundamental.
Mention is nevertheless often made of "epidrugs" which could help to cure certain cancers. What is the situation here?
E. H.: In all cancers, the distribution of epigenetic modifications is abnormal. For a long time, it was even postulated that the genes involved in the initiation of a tumour were epigenetically modified. Hence the interest in epidrugs : these molecules, which have been known for several decades (they were used in chemotherapy even before we understood how they functioned) do indeed act on epigenetic modifications, and in particular on the best known of them, DNA methylation. Thanks to the high-throughput sequencing of tumour genomes, we have found out that most tumours are due to mutations that directly affect the DNA sequence of so-called “driver” genes; in other words, those that lead to the development of a tumour. What’s surprising is that some of these mutations also affect the genes involved in epigenetic processes. This explains the generalisation of epigenetic modifications in tumours. The problem with cancer is that nothing is simple: genes are mutated, the genome is altered, and so is the epigenome, but we still don’t know whether these changes are related and in which direction they occur. The use of epidrugs also brings up a number of issues, as they do not only target one or two genes but all the epigenetic marks of an individual, with consequences that we cannot fully control at this stage. This is where we stand at present on cancer and epigenetics. It is a research field that raises high hopes but is not progressing very rapidly. Once again, it requires an enormous amount of basic research. Unlike the genome, which has been completely deciphered, we still don’t know everything about the epigenome, particularly as far as cancer is concerned.

You are known throughout the world for your work on X chromosome inactivation. Can you explain what this is about?
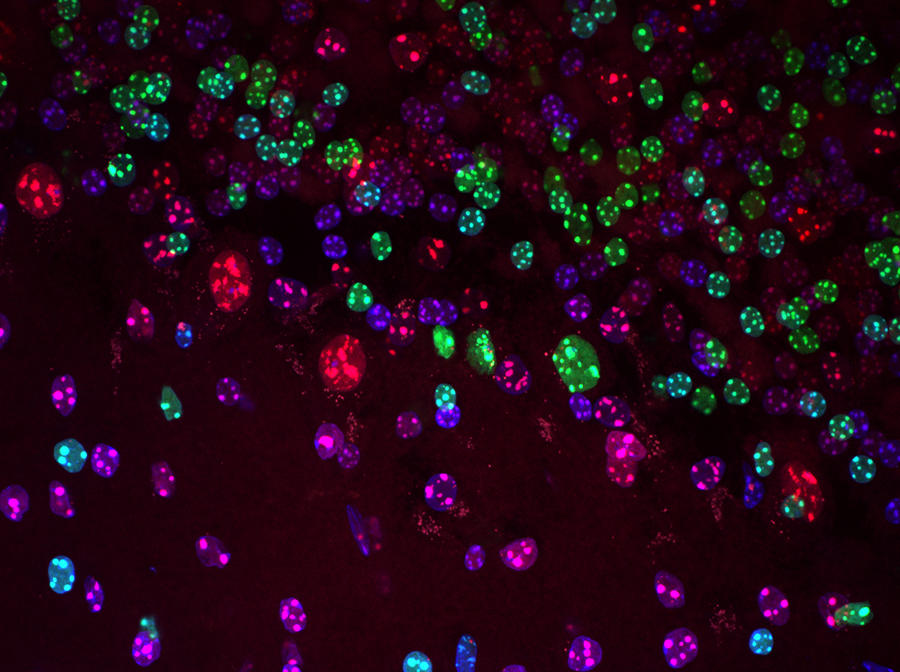
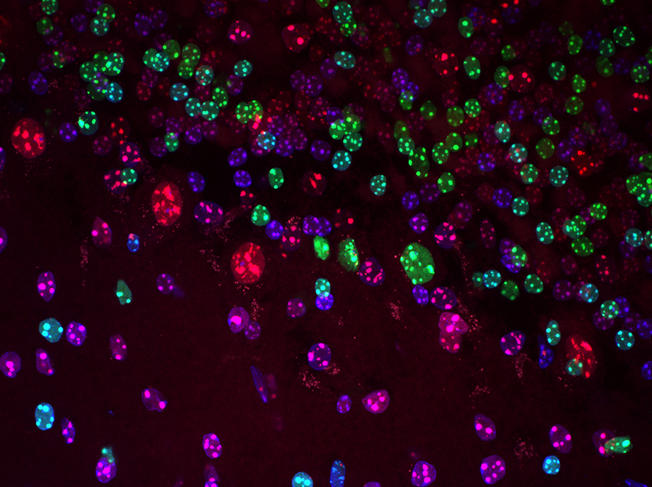
E. H.: As you know, female mammals carry two X chromosomes inherited from each of their parents, while males have a Y gene from their father and an X gene from their mother. The problem is that the Y chromosome carries very few genes, barely a hundred or so, which are important for masculine sex traits, and the X one carries more than a thousand! To compensate for this imbalance between males and females, a process that inactivates one of the two X chromosomes has therefore developed in females. This is a 100% epigenetic programme that deletes a whole chromosome!
What in practice are the implications of this X chromosome inactivation?
E. H.: In most mammals, including humans, the choice of the X chromosome to be inactivated is totally random from one cell to another during development. This means that females are a real mosaic for the expression of X genes. In each tissue (brain, blood, kidneys, etc.) the proportion of cells that activate the paternal X rather than the maternal X may differ, and this also varies from one individual to another. Even monozygotic twins (from the same egg) are not identical in this respect.
This has some very real consequences. For example, we know that genes carried by the X chromosome are involved in certain neurological diseases, including Rett syndrome severe autism which compromises the survival of those affected. This syndrome is due to the mutation of an X gene: boys who carry this mutation die very young because their brain functions poorly. Girls, on the other hand, react differently, depending on the proportion of mutated and healthy X chromosomes expressed in their cells. What I’m hoping, together with my team, is that we will manage to reactivate the silent X genes by manipulating the epigenetic marks and help find a cure for these women one day. But we still have a long way to go.
Since January 1st, you have been the new director general of the European Molecular Biology Laboratory (EMBL). Does this mean that you are going to put your research on X chromosomes on hold?
E. H.: Absolutely not. I have moved to Heidelberg, the headquarters of the EMBL, with six members of my old team at the Institut Curie who accepted to come with me to Germany. By asking me to head this organisation, the committee decided to choose a scientist who is still actively conducting research work and will continue to do so during her term of office, which is renewable every five years.
Why did you accept this job at the EMBL?
E. H.: The EMBL is an intergovernmental organisation that was set up in 1974 to support research in molecular biology in Europe and keep scientists on European soil. It now includes 29 countries and has a dual role: to perform basic research through its network of 1700 scientists and six institutes based in Heidelberg and Hamburg (Germany), Barcelona (Spain), Hinxton (near Cambridge, UK), Rome (Italy), and Grenoble (France); and to provide all researchers in its member countries with cutting-edge technologies for the storage and analysis of data, etc. Heading up this organisation is of course an honour, which I accepted for three reasons. Firstly, the EMBL focuses on basic research and excellence: this is essential for research in biology to progress. Secondly, when an opportunity of this type is offered to a woman, I believe it is important to accept; if women do not make the effort, whatever the sacrifices, then things will never change! The third reason is what is happening in Europe. The Greek crisis and the vote for Brexit have severely weakened this wonderful venture. I am European through and through: my father is British, my mother Greek, I am married to a Frenchman and I have worked in France since the beginning of my career. I must do something for Europe!


What will be your mission at the head of this organisation?
E. H.: The EMBL is a flexible, proactive structure that adjusts its research priorities on a regular basis. A new research programme is indeed implemented every five years. The current scheme, which runs until 2021, is called Digital Biology and its focus ranges from the functioning of the single cell to that of the entire organism. Once in Heidelberg, my first task will be to think about the next multiyear programme. This will require considerable diplomacy and consultations with our member countries. I would like to point out that the EMBL is independent from the European Union, and that the UK will continue to be part of it after Brexit. Similarly, the foreign researchers who work at our unit in Hinxton, near Cambridge, will not be affected in any way.
You are British. How is your personal situation going to be affected by Brexit?
E. H.: I told you, I am profoundly European—and upset at the idea of Brexit. Nevertheless, I need to prepare for it, and this is why I am currently trying to obtain French nationality, having lived in France for thirty years.
Since 2017 you have been co-director of the Pause programme managed by the Collège de France, where you have been a professor since 2012. This project, initiated by the previous government, consists in giving a better welcome to foreign scientists living in exile. What are the reasons for this commitment?
E. H.: I have an international culture and have worked almost all my life in France without anyone ever asking any questions. I am also a scientist—a profession where you travel the world and collaborate with people of all nationalities. I find it hard to accept that my colleagues may no longer have this freedom. There have been induction programmes for foreign scientists in the US and the UK for many years, but nothing in France. The Pause project aims to make up for this, allowing scientists at risk in their own countries to pursue their work in France in the best possible conditions. Usually, they already have contacts here, in universities and research laboratories. The idea is to help them settle in through financial incentives for the higher education or public research institutions that wish to host them, but also to help them with formalities for obtaining a visa or residence permit and finding somewhere to live in France. So far, 140 scientists from Syria, Turkey, Iraq, Yemen, Venezuela, Afghanistan or Burundi, for example, have been able to benefit from this programme, and I am proud to be able to help promote it.