You are here
Fighting HIV on All Fronts
Since it was discovered in 1983, the status of HIV has evolved considerably. A fatal condition during the 1980s, the introduction of triple therapies in 1996 meant that AIDS—or acquired immunodeficiency syndrome—has become a chronic disease for individuals with access to treatment (i.e. half of the 37 million seropositive patients throughout the world). This combination of at least three antiretroviral compounds has enabled the effective control of one of the main characteristics of this virus: its extreme mutability. Better still, by preventing seropositive individuals from transmitting HIV, triple therapies are a preventive mechanism in their own right.
HIV is a retrovirus1 that infects certain cells in the immune system of the host organism, especially CD4+ T-cells, which it uses to proliferate and spread. After several years (up to ten in some cases), the viral load (the number of viral copies in the blood) shoots up and the T-cells that protect the organism from external attack are massively destroyed. Hence the individual’s high susceptibility to certain cancers and opportunistic infections (tuberculosis, Kaposi’s sarcoma, etc.) which are normally controlled by the immune system.
Twenty years after the introduction of triple therapies, the arsenal of available antiretroviral treatments (between 25 and 30 compounds), their improved efficacy and reduced side effects mean that most treated patients can live normal lives. Thanks to antiretroviral agents, viral replication is blocked and the viral load is kept below 200 copies per ml of blood. Beneath that threshold, the risk of associated diseases and viral transmission is infinitesimal. As a result, patients diagnosed early and who comply with the treatment—one tablet per day, for life—have the same life expectancy as that of the general population.
Tracking reservoir cells
Nevertheless, there is no permanent cure as yet. Complete eradication of the virus in seropositive individuals is now the prime target for scientists. For this purpose, they are focusing on a process specific to HIV: its dormancy, as from the early hours of the infection, in certain cells of the immune system referred to as “reservoirs.” This veritable archiving of the virus, even while it continues to replicate, is specific to HIV, and what makes it so difficult to control. "A cure for AIDS will only be possible if we manage to control these viral reservoirs, either by eliminating them or by preventing them from expressing the virus," explains Monsef Benkirane, director of the Institute of Human Genetics (IGH)2 in Montpellier (southern France).
When a seropositive patient is given antiretroviral therapy, the virus is indeed no longer detected in the blood, but remains present in these reservoirs in a precursor (pro-virus) form that is undetectable by the immune system and not susceptible to treatment. Hence the rebound in viral load following discontinuation of the treatment, and the obligation for patients to take the medication daily, for a lifetime.
Several strategies are being investigated to deal with these reservoirs. One of them consists in reactivating the "hidden" virus to make it visible to the immune system, which can then control it better. This option necessitates a prior understanding of the molecular mechanisms that regulate how the virus is “put to sleep” and then reactivated. Although knowledge of these processes has considerably improved in recent years, the resulting clinical trials have not been conclusive. Another, more futuristic approach currently being considered would be to remove the viral DNA integrated into the DNA of reservoir cells using gene therapy and notably new technologies such as CRISPR, “molecular scissors” that can selectively cut out certain genome sequences.

The third lead aims to kill the reservoir cells, but to achieve this, they must be differentiated from healthy immune cells. It is in this field, which mobilizes numerous teams throughout the world, that Benkirane and his colleagues at the IGH achieved a decisive step forward3 in the spring of 2017 by identifying the first surface-specific marker of reservoir cells. This result points to the possibility of directly isolating these reservoir cells in patients, and eventually deploying new therapeutic strategies.
A vaccine: some promising leads
A definitive cure for AIDS is not the sole objective. Not contracting it is another. After years of research and false announcements, many scientists are still striving to develop a preventive vaccine. “The principle of conventional immunization based on injecting an attenuated form of the virus so as to stimulate the immune response is ineffective in the case of HIV because of its considerable ability to mutate (the virus is present in numerous versions),” explains Vincent Vieillard, a researcher at the CIMI4 in Paris. “To develop a vaccine, we need to devise completely new strategies.”
A clinical trial involving nearly 6000 people is currently underway in South Africa. Yet it concerns certain types of the virus present exclusively in southern Africa—and would only guarantee limited efficacy (up to 50% at best). Other leads are promising though, such as the so-called neutralizing antibodies found in a small percentage of patients, which can control numerous versions of the virus at the same time. In the US, trials involving the direct injection of these antibodies have produced satisfactory results in monkeys and humans.
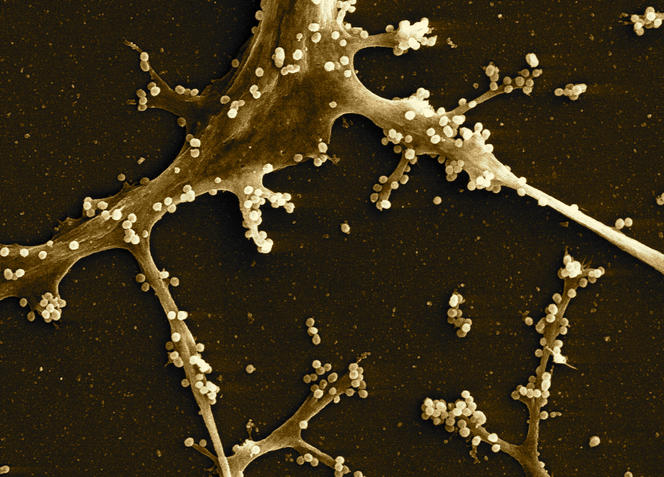
“The challenge today is to be able to generate these antibodies in healthy individuals to protect them against infection,” explains Arnaud Moris, another researcher from the CIMI. To achieve this, the scientists are studying the interactions between these antibodies and the viral envelope in order to design immunogens. These molecules simulate proteins in the viral envelope and—when identified as foreign elements by the body—trigger an immune response and the production of antibodies. Some of these immunogens are the subject of clinical trials.
The vaccination strategy is also being evaluated in combination with antiretroviral therapy in order to boost the immune system of infected patients: this so-called therapeutic vaccination could compensate for the reduced treatment efficacy observed in a small fraction of patients.
Finally, still in the vaccine field, scientists are studying another population of “unusual” patients: the "elite controllers" (who make up less than 1% of patients). Although infected and untreated, they naturally control the infection by maintaining a viral load that is durably undetectable. Numerous studies seek to explain the specific mechanisms of this exceptional immunity, which in the future might give rise to new tools for vaccination.
At the heart of molecular mechanisms
To conquer HIV, scientists are also trying to elucidate the interactions between the virus and the cells it infects—or with the immune system. They have a dual objective: to improve fundamental knowledge of biological processes at the molecular scale and to define new therapeutic targets. “If clinical trials designed to reactivate the hidden virus are generally floundering, it is because we have reached a limit,” states Stéphane Emiliani, a researcher at the Institut Cochin.5 “We are confronted with a serious lack of knowledge on the molecular mechanisms of the virus and particularly those that govern its integration, dormancy and expression.” This is a real challenge if we consider the heterogeneity of the modes of integration and expression that HIV is able to develop during its mutations.
With his team, Emiliani is studying different stages in the replication cycle of the virus, and notably insertion and expression of the viral genome into cellular DNA. Scientists have thus identified the protein in the host cell that interacts with the viral enzyme (integrase) and enables this insertion. Based on their patented findings, a biotechnology firm has developed new molecules that block the interaction between integrase and this cell protein. An alternative to existing antiretroviral compounds which block the activity of the enzyme, this strategy—currently under preclinical study—could be a weapon to fight resistance due to mutability of the virus.
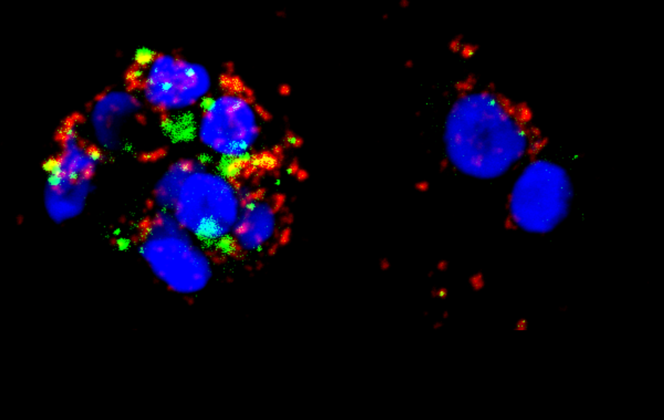

Concerning the immune system, research teams are specifically targeting molecules that are produced naturally to combat HIV: interferons, which are potent antivirals. The problem is that, when they are produced over a long period of time—as in chronic autoimmune diseases and AIDS—these protective agents eventually destroy other cells in the immune system (lymphocytes, etc.). Hence the idea, which is gaining traction amongst scientists, to interrupt their production. “Treating patients in the chronic phase of AIDS using anti-interferons is becoming a real therapeutic strategy,” comments Jean-Philippe Herbeuval, from the Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques.6 With his team, the researcher has just obtained some very promising results7 by testing molecules—namely histamine monoamines—that are able to partially inhibit cells specialized in interferon synthesis. Combining existing triple therapies with the intermittent blockade of interferons would allow a reduction in antiretroviral therapies and help to limit their adverse effects, as well as counter viral resistance problems and strengthen the control of opportunistic diseases.
Research on transmission of HIV is also being pursued. Transported via breast milk, blood and genital secretions, the virus is present in these fluids either in a free form or integrated into the cells it has infected. Numerous studies are notably focusing on viral synapse formation, the close contact that is created between infected cells and the surface of the genital mucosa, ensuring rapid internalization of the virus towards the inner layers of the mucosa. The challenge is to elucidate the functioning of this synapse with a view to designing local strategies to block the virus at its portal of entry into the body.
Mucosae under the spotlight
Furthermore, scientists are seeking to improve their understanding of the mechanisms of immunity at the level of the mucosa, especially in the case of individuals who are regularly exposed to the virus (for example, prostitutes) but nevertheless remain seronegative. “We are unable to fully explain this phenomenon,” comments Morgane Bomsel, a researcher at the Institut Cochin. “Perhaps the fluid is little infective during initial contacts, and these people become immunized through repeated intercourse. This question demonstrates the usefulness of studying the immune response at the local—as well as whole-body—level.” By examining the antibodies (immunoglobulins A) specific to HIV and present in the vaginal mucosa, Bomsel’s team has succeeded in developing a candidate for a preventive vaccine. Administered via the intranasal route to the mucosa, it induces a protective immune response in the vaginal and rectal mucosae (which was not the case using traditional intradermal vaccination) AND in the blood. Based on this novel strategy, the vaccine has successfully completed its recent phase-I clinical studies.8
The AIDS virus has not yet revealed all its secrets. Experts are however unanimous on one point: as well as the benefits already obtained regarding disease control, the progress achieved in HIV research has revolutionized fundamental knowledge in biology, immunology and virology. A good example was the discovery only ten years ago of intracellular immunity, which establishes that each cell in the body is intrinsically equipped to resist infections and facilitate the response of the immune system. “The complexity of HIV, its exceptional ability to adapt and constantly develop new stratagems to circumvent the body’s defenses make it necessary to explore the fundamental mechanisms that govern the immune system ever further,” Moris concludes.
- 1. These viruses possess an enzyme called reverse transcriptase, which translates viral RNA into DNA and thus allows it to be inserted in the DNA of the cells it infects.
- 2. CNRS / Université de Montpellier.
- 3. Publication in Nature, March 2017.
- 4. Centre d’Immunologie et des Maladies Infectieuses (CNRS / UPMC / Inserm).
- 5. CNRS / Inserm / Université Paris-Descartes.
- 6. CNRS / Université Paris-Descartes.
- 7. Work published in Nature Communication, May 2017.
- 8. Phase-I clinical studies are mainly designed to evaluate the safety and tolerance of a vaccine in humans.
Explore more
Author
Stéphanie Belaud has been a scientific writer for more than ten years. She specializes in biology, medicine and the environment.