You are here
The endless cycle of pandemics
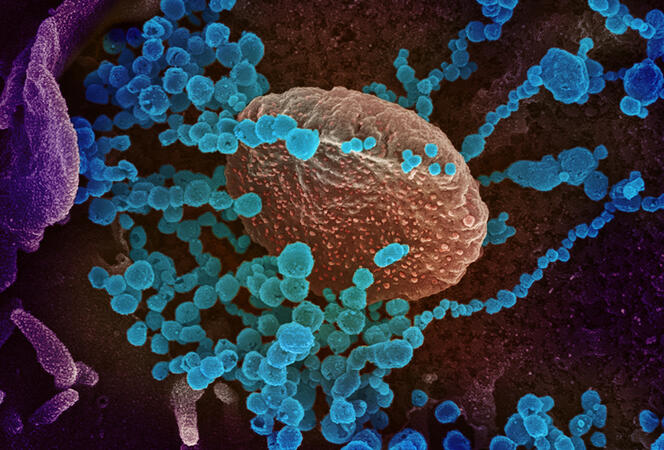
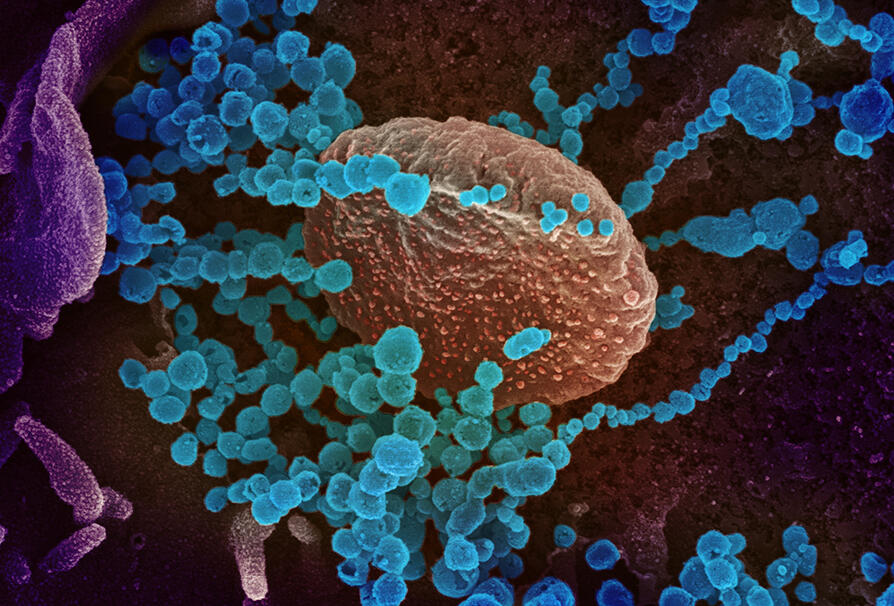
Epidemiologists are once again on high alert. In recent months, thousands of sea lions have been found dead on the beaches of Chile and Peru. The cause of death: H5N1, or bird flu, a virus that has been under close scrutiny for 20 years. Since its re-emergence in China in 2003, there have been fears that it could cause a full-scale pandemic. To reach that point, the virus lacks only one thing: the ability to pass efficiently between humans. Until now, most human cases of this strain have resulted from contact with infected birds.
This is why the sudden death of so many sea lions is worrisome. The mortality rate may suggest that bird flu has spread from one individual to another. “If this is confirmed, it would be of utmost importance,” warns Martin Blackledge, at the IBS1. “It would mean that the virus is adapting to mammals.” And suddenly the memory of the annus horribilis of 2020 springs to mind…
The end of optimism
Long gone is the golden era of the 1950s and 1960s, when doctors and health authorities in developed countries believed that the threat of infectious diseases would soon be a thing of the past. “We saw progress in hygiene, hospital infrastructure, vaccines and antibiotics. We had just scored a huge victory over polio,” recounts Serge Morand, a research professor at the MIVEGEC2. “It was all settled, we’d understood everything, and it was time to move on,” he adds, with a note of irony.

The dream was short-lived. A series of unknown bacteria and viruses – HIV, Legionella pneumophila (Legionnaires’ disease), Ebola, Nipah, Sars – put an end to this blissful optimism. The experts had failed to consider the incredible adaptability of microorganisms in response to any human intervention. They had also neglected important factors that favour the emergence of new pathogens, such as the development of trade and air transport, as well as the inexorable degradation of the planet’s ecosystems.
Expanding agriculture and pandemics
The emergence of the Nipah virus is a textbook case. It unites all of the key factors that in recent decades have exacerbated the risk of a pandemic: the expansion of the agricultural frontier, intensive livestock farming and the accelerated movement of people and goods. The pathogen originated in the Malaysian village of Nipah, where in 1998 a population of bats had settled near a pig farm. New oil palm plantations had deprived them of their habitat and food source, and the mango trees around the hog pens became their new feeding ground.
These bats, a species of flying fox, were the natural reservoir of a previously unknown Henipavirus. Their droppings infected the pigs, which in turn contaminated the livestock workers. An epidemic broke out, with some 300 cases causing more than 100 deaths, preceded by acute encephalitis. Exports of meat and live hogs then allowed the Nipah virus, as it was later called, to spread throughout Southeast Asia. Fortunately, it does not pass very efficiently from human to human, although it still kills a few dozen people a year.
This event clearly shows the danger posed by zoonoses (animal diseases), since 75% of all emerging pathogens come from animals. Other recent examples include HIV (from chimpanzees, gorillas and mangabeys), Middle East Respiratory Syndrome (dromedary camels), Ebola, Sars-CoV-1 and Sars-CoV-2 (bats).
Intensive livestock farming, a breeding ground for zoonosis
That is where livestock farming comes in. “Livestock animals are intermediate hosts that serve as pathogen amplifiers,” Morand explains. Indeed, herds of animals, all genetically similar and often under stress due to poor living conditions, act as vast bioreactors in which natural selection carries out some astonishing biological experiments.
A paradigmatic case is Mexican flu, also called swine flu, which shook the world in 2009. All the evidence indicates that the strain originated at a pig farm in Mexico. This highly contagious virus has one quite surprising characteristic: its genome contains elements of avian, human and porcine strains. The emergence of this strange chimera required a series of infections and superinfections that could only have occurred in an environment concentrating thousands of animals.
The leap from animals to humans subsequently becomes easier. Strains that have been “perfected” on farms can infect wildlife, which then migrates, spreading contagion throughout the world. And livestock farming has been expanding continuously in recent decades: today there are some 1.4 billion cows, 1.5 billion pigs and about 40 billion chickens worldwide. To feed all those hungry mouths, crops have had to be expanded as well, further encroaching on forests and other wild natural areas. This change in land use is also conducive to pandemics. “Deforestation, crop expansion and landscape simplification result in more frequent contact between wild and domestic fauna,” Morand points out. The Nipah virus stands as proof. However, another phenomenon is also at work. In healthy ecosystems with rich biodiversity, ecological balance including predation and competition prevents any single species from experiencing a sudden population boom. The spread of pathogens is more difficult when their hosts are few and far between.
Everything changes when the ecosystem enters a phase of crisis. “When an environment is degraded, it loses predators and competitors, allowing certain species to multiply exponentially,” Morand explains. These species become invasive, along with their pathogens, spreading out and inevitably coming into contact with humans and their domesticated animals.
Climate change is another factor that favours the emergence of pathogens. It puts ecosystems under pressure, pushing wild species out of their natural habitats and expanding the endemic zones for virus carriers like mosquitoes or ticks.
The fragile species barrier
For an animal pathogen, adapting to humans is no easy process. “It has to mutate in order to exploit human cells,” Blackledge says. “It must ‘convince’ its host’s proteins to work for it.” Even so, the species barrier is not insurmountable. The scientist studies the mutations that allow the H5N1 bird flu virus to infect human cells. As part of a collaboration with the Institut Pasteur and the European Molecular Biology Laboratory (EMBL), he examined the interactions with the host of a key viral protein: polymerase. The latter enables the massive copying of the virus’s RNA, triggering the production of viral proteins. The team of researchers is investigating the mechanisms by which the substitution of a single amino acid in polymerase allows the avian virus to multiply in human cells.
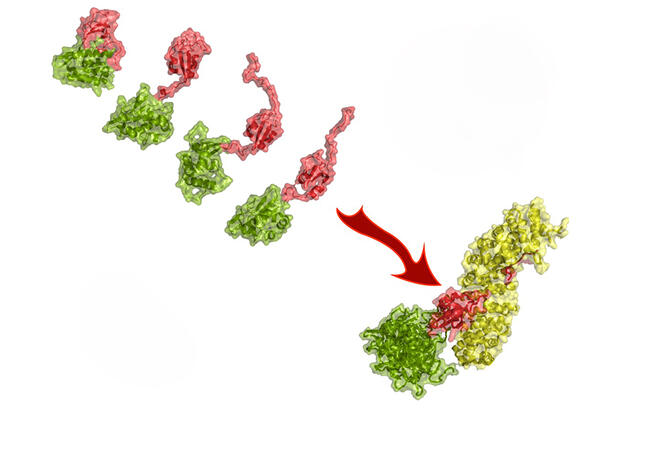


The mutation that makes this substitution possible does indeed exist in nature. Although necessary for the virus to infect humans, it is not sufficient to turn H5N1 into a human pathogen. Why H5N1 is not transmitted from human to human is still an open question. But considering what happened with the sea lions, it remains to be seen whether this inability will last.
The microbial arms race
“Since the appearance of modern humans, there have been about 7,500 generations of our species. The HIV virus reaches the same number of generations in an infected person after 20 years without treatment,” reports Samuel Alizon, research professor at the CIRB3, in his book C’est grave, Dr. Darwin?4 And of course, each new generation is an opportunity for genetic mutations to produce yet another variant. As a result of this agility, pathogens are a moving – and fast-reacting – target: any human intervention to combat a virus, bacterium or fungus selects the variants that are most likely to resist that intervention. A strain can thus become more virulent or contagious, or can acquire forms of resistance that make it very difficult to treat. It can also become “invisible” to the immune system, or reduce the time it needs to reproduce – all due to the blind power of natural selection, which chooses the variants that replicate most efficiently.
It is often difficult to predict how each characteristic will evolve. Virulence, for example, is “related to the fact that a pathogen exploits its host’s resources, and that this exploitation comes at a cost for the host”, Alizon explains. “Generally, the variants that make the best use of those resources reproduce the most efficiently.” Virulence is the result of an eminently Darwinian equilibrium. A strain that does not exploit its host as well as a neighbouring one does is likely to be replaced. At the same time, if it kills its victim too quickly, it won’t be able to spread.
From a scientific point of view, Covid-19 has offered an extraordinary observatory for pathogen evolution. “We have seen how the virus’s capacity to adapt plays a key role in the epidemic process,” comments the CIRB researcher François Blanquart. The succession of variants has been an especially rich source of information. “With the variants, we have seen an extraordinary dynamic of selective replacement. Each new variant displaced the previous ones in record time.” The Alpha variant, more virulent and more easily transmissible than its predecessor, supplanted the original Wuhan strain. It was soon followed by Delta, even more harmful and contagious than Alpha, which drove its competitors to extinction.
The arrival of vaccines altered the course of the virus’s evolution. “Omicron eliminated the earlier strains in part because it spread very easily among vaccinated populations,” Blanquart explains. Scientists believe that in the future the coronavirus, like the flu, will have many variants circulating simultaneously, evolving in a highly diverse immune landscape. In any case, it will be impossible to eradicate completely. Homo sapiens is now its natural reservoir.
The fungal resistance movement
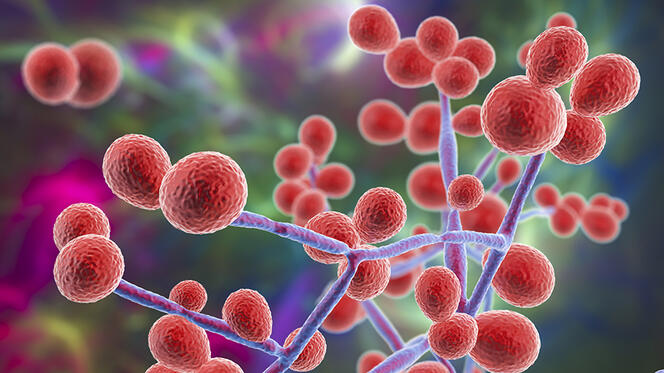
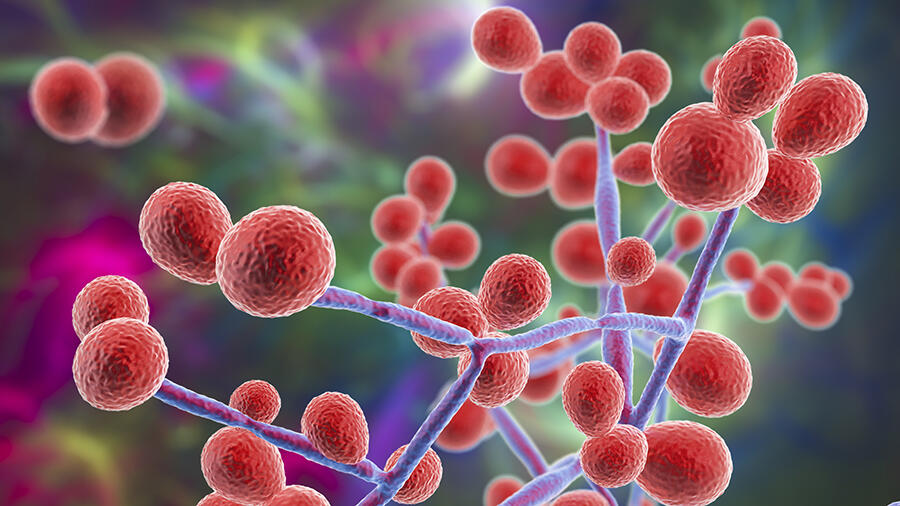
In October 2022, the World Health Organization (WHO) warned of a growing threat: fungi. The international organisation released a list of 19 species for priority monitoring and called for more intensive scientific research on these often-overlooked pathogens. “In the past ten years we’ve been seeing new species emerge,” reports Sarah Dellière, a researcher at the Institut Pasteur and a medical microbiologist at Saint-Louis Hospital in Paris. “This could be linked to climate change, which also causes an evolution in the distribution areas of certain known fungi.” Among these emerging strains, Candida auris is worthy of close attention. Discovered in 2009, this yeast is harmless when it colonises the skin, but when it enters the bloodstream, often as the result of a medical procedure, it proves fatal in half of all cases.
The natural habitat of Candida auris appears to be the shorelines of estuaries and mangrove swamps. It is highly resistant to heat, chemical agents like chlorine and most fungicides. These characteristics suggest that before becoming a hospital pathogen it must have developed the capacity to withstand pollution, antifungal azoles used by farmers and rising temperatures linked to global warming. The fungus is so robust that when it appears in a hospital, as it did in Valencia, Spain in 2015, it is very difficult to eradicate.
Another example of a fungus whose resistance is cause for concern in the hospital world is Aspergillus fumigatus, a mould that is ubiquitous in the environment and poses a danger to people with weakened immune systems. “In the Netherlands, where the tulip fields are sprayed with fungicides,” Dellière says, “10% of the patients who come to the hospital with Aspergillus infections have a resistant strain.” The only viable treatment is a last-line antifungal that has highly toxic effects on the patients. Here again, health risks are exacerbated by environmental degradation and the extraordinary adaptive capacity of microorganisms.
And the same holds true for bacteria: constantly exposed to the antibiotics used in medical treatments and by livestock farmers, they have learned to resist. Or rather, to be precise, exposure has selected the resistant strains. Described as the silent pandemic, antimicrobial resistance by pathogenic bacteria threatens to bring humanity back to the days before penicillin, when commonplace infections claimed countless lives.
Anticipating the next wave
Can a repeat of the tragedy of 2020 be prevented? Is a world without pandemics conceivable? Bill Gates, the former Microsoft CEO turned philanthropist, believes it is, provided his latest brilliant idea is put to use: a team of hyperspecialised virus hunters ready to go anywhere at the first sign of an outbreak. If only things were that simple… “We think of epidemics as something that emerges and spreads quickly,” Alizon explains. “This was the case with Sars-CoV-2. But there are also cases of much slower pandemics, like antimicrobial resistance. Some infections, such as HIV, are chronic, and it takes years for the symptoms to appear. By the time they’re detected, the initial outbreak is long over.”
To reduce the threat of a pandemic, the answer lies in plain old public policy. The first step is improving healthcare systems, especially in Southern Hemisphere countries where access to medical treatment is not always a given. “Socioeconomic conditions affect the impact of pandemics,” Alizon cautions. “For example, analyses have shown that the insufficiency of healthcare systems in China may have helped spread Sars-CoV-2.” Another important point is improving security at Biosafety Level 3 and 4 laboratories, those that handle dangerous pathogens. Leaks are known to be a possibility.
“The most famous example is the ‘Russian’ flu of 1977, which killed about 700,000 people,” the researcher points out. “This variant is so close to the Spanish flu of 1918 that the hypothesis of human error is highly likely.”
For Sars-CoV-2, there is a scientific consensus that the pandemic originated in the Wuhan market. As for how the virus got there, the majority of scientists lean towards a natural origin. For others, however, the possibility of a laboratory leak cannot yet be ruled out.
In terms of prevention, a new proposition is gaining ground: the One Health approach. Championed by major international bodies such as the United Nations Food and Agriculture Organization (FAO) and the WHO, it is based on the premise that the health of human populations is closely linked to that of ecosystems and farm animals. Any damage suffered by one is a threat to the others. Morand would like to demonstrate that the corollary is also true: any action to protect the environment has a positive effect on human health. To prove it, he is studying the consequences of reforestation on the health of the populations of 14 communities bordering a national park in Thailand’s Nan Province. “Our preliminary findings indicate that the wildlife species that adapt to humans, such as rodents, are in decline,” the scientist reports. “And it seems that the infection rates of leptospirosis and scrub typhus are decreasing.” Therefore, the best way to reduce the risk of a pandemic is to undertake actions that can slow the erosion of biodiversity, reduce pollution, limit the impact of agricultural systems and mitigate climate change. ♦
-------------------------------------------------
The plague: an ever-present threat
Yersinia pestis is still to be feared. The bacillus that causes the bubonic plague, the same that killed 30 to 50% of Europe’s population in the 14th century, is one of the 16 pathogens on the priority monitoring list of ANRS Emerging Infectious Diseases, an independent agency of the Inserm.
“The plague has a singular way of reappearing in places where we thought it had disappeared,” says Sébastien Bontemps-Gallo, a researcher at the Centre for Infection and Immunity of Lille (CIIL)5. “It recently cropped up again in Algeria and Libya, and a few dozen infections occur in the US every year. In China, the occasional resurgence forces the lockdown of entire cities.” But the biggest threat today is in Madagascar, where the ricefield rat and its fleas provide a natural reservoir that allows it to stay in circulation. Although usually not a major problem, it can still spark epidemics, including one in 2017 that killed 171 people in a few months.
Like all pathogens, Yersinia pestis evolves. “In Madagascar we have found strains that are multi-resistant to antibiotics,” Bontemps-Gallo reports. A good reason not to lose sight of the microbe. The researcher is investigating the molecular mechanisms that enable the bacterium to adapt to its vector, the flea, with the goal of identifying therapeutic targets for a possible vaccine or drug treatment.
-------------------------------------------------
A new laboratory to study the links between environment and health
In Bangkok on 12 July, the CNRS, Mahidol University and Kasetsart University formalised the creation of an international research laboratory (IRL) called Health, Disease Ecology, Environment and Policy (HealthDEEP). This French-Thai laboratory will conduct research on biodiversity and its connections to human health. It will serve as a centre not only for collaborative research but also for training and dialogue with policy-makers (see press release).
For further reading on our website
Fighting antibiotic resistance
The origin of SARS-CoV-2 is being seriously questioned
Choosing a vaccination strategy to deal with Covid variants
- 1. Deputy Director of the IBS (Institut de Biologie Structurale – CNRS / CEA / Université Grenoble Alpes).
- 2. Maladies Infectieuses et Vecteurs : Écologie, Génétique, Évolution et Contrôle, (CNRS / IRD / Université de Montpellier).
- 3. Centre Interdisciplinaire de Recherche en Biologie (CNRS / INSERM / Collège de France).
- 4. “Is it Serious, Dr. Darwin? Evolution, Microbes and Us”, Samuel Alizon, Seuil, February 2016 (in French).
- 5. CNRS / CHU Lille / INSERM / Institut Pasteur Lille / Université de Lille.