You are here
Mechanobiology exerts creative pressure

After a cut or scratch, the integrity of the skin is broken and the body has to trigger a whole battery of processes to start healing. To achieve this, cells need to migrate, weave in and out, touch and proliferate until they fill up the spaces where the skin is missing. These cellular movements involve the intervention of essential mechanical actions. Long overshadowed by the millions of chemical reactions that drive the complicated machinery of the body, these phenomena nevertheless play a crucial role in the living world. Their study, or mechanobiology, is now benefiting from numerous technological advances, notably in microscopy.
“When scientists started to study cellular imaging and embryology in the early 20th century, they already thought that geometry and mechanics played an important role in cell physiology, division and migration,” explains Nicolas Minc, CNRS research professor at Institut Jacques Monod1. “But with the discovery of DNA in 1953, and the popularisation of genetic approaches, these ideas were set aside for several decades. It was only in the late 1990s, following numerous advances in the field of microscopy, that the mechanical signals perceived by cells were once again taken into account.”
From division to migration
Indeed, the precision of microscopes has evolved so much that they can now “see” into a tissue through several micrometres of thickness. Some, such as atomic force microscopes (AFM), have probes that enable them to measure the rigidity of the membrane or that of a particular constituent of a cell. Traction force microscopes (TFM) can evaluate the forces exerted by a moving cell on its immediate environment. All these tools have made it possible to study the movements and forces within cells revealing, quite unexpectedly, that these can not only detect mechanical signals but also emit them. A whole aspect of the functioning of living beings therefore remains to be investigated.
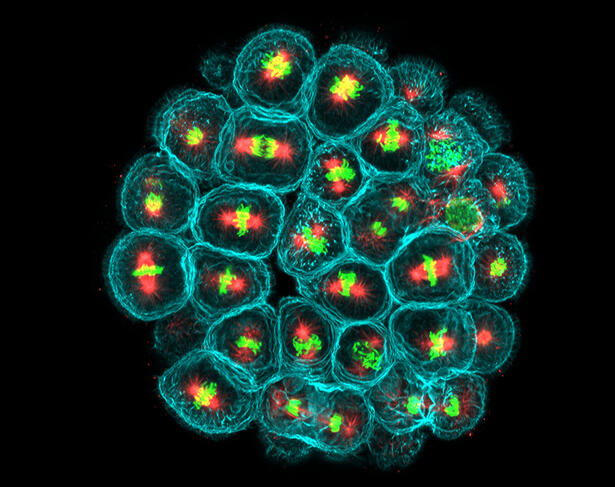
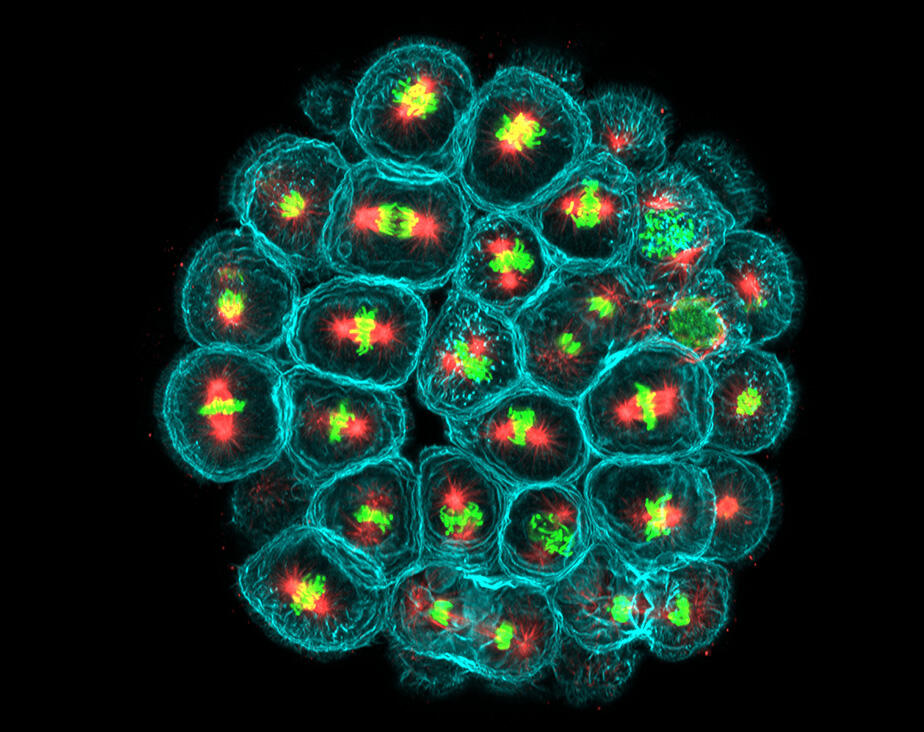
For the past ten years or so, Minc has been studying the impact of mechanical forces on the geometry and division of embryonic cells in the sea urchin, an animal that has the advantage of producing gametes (male or female reproductive cells) in large quantities. Like all eukaryote cells – possessing a nucleus – those in sea urchin embryos also contain numerous fibres, or microtubules, which assemble into a network, the mitotic spindle. During the development of this network, genetic material is divided equally between mother and daughter cells. Minc and his team have designed an original system where they attach tiny magnetic beads to the mitotic spindles in order to control their position in the cells and to measure the forces at play.
“We knew that the fibres exerted pressure within the cell, but did not understand their importance,” explains Minc. “Our invention means that we have been able to measure this pressure for the first time. The figures thus obtained then served to build models of force generation at the scale of hundreds of thousands of microtubules.” This patented system indeed enables different mechanobiological investigations, such as mechanotransduction, the phenomenon whereby cells – notably thanks to receptors on their surface – detect external mechanical forces and constraints and ultimately translate them into biochemical and genetic signals. These forces may arise not only from the environment but also from nearby cells.
“This makes it possible to transmit messages in a linear manner,” details the scientist. “For example, these signal cascades can guide cell migration and other collective behaviours.” The researchers at Institut Jacques Monod are also applying their magnetic system to the study of organoids, miniature artificial organs developed for the in vitro study of different pathologies, and this work has indeed enabled a clearer understanding of how intestinal cancers affect cell division and migration.
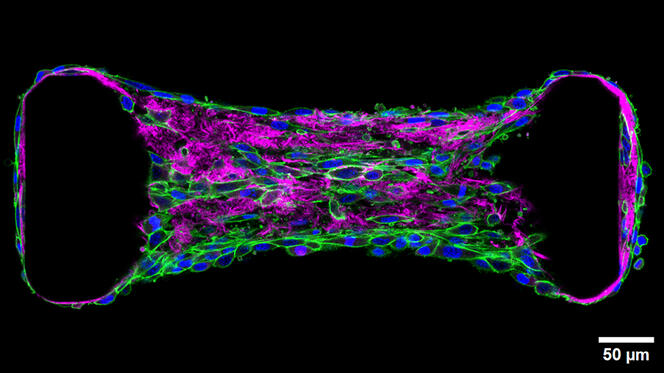
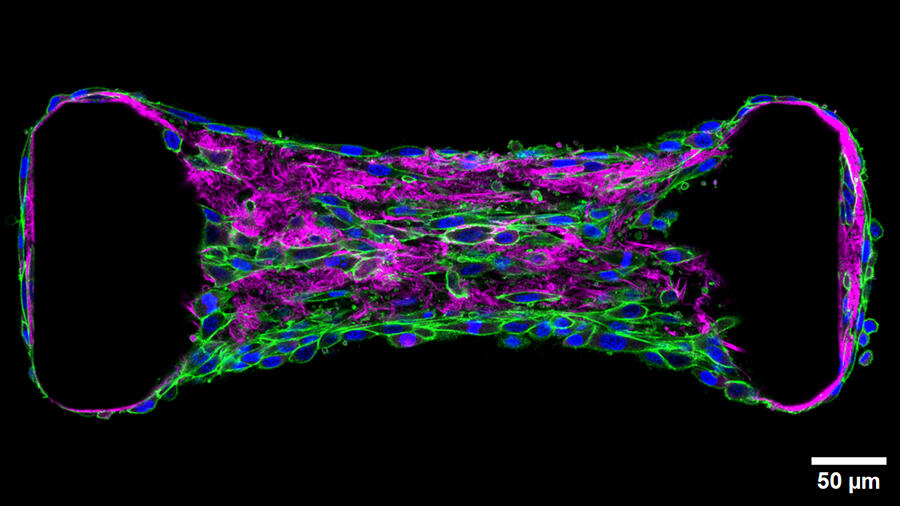
Migration occurs notably within, on and through the extracellular matrix (ECM), a scaffold of macromolecules mainly consisting of collagen synthesised and structured by specialised cells (fibroblasts). The site of much cellular traffic, the ECM is currently the focus of particular attention from scientists such as Thomas Boudou at the Interdisciplinary Laboratory of Physics (LIPhy)2 who is exploring the mechanobiology of tissues. “These cells exert continuous forces on their environment and their neighbours,” he explains. “The extracellular matrix can be compared to a cobweb that allows mechanical signals to propagate along its fibres. This information can then drive cells to migrate or differentiate.”
To study the mechanical forces involved in cell migration or differentiation, the scientist uses optogenetics: a method that, after genetic modification, enables activation of a specific protein thanks to light. Like a waterfall, this activation triggers another, which ultimately induces the biological process of interest. Boudou thus transforms cells into true levers that he can contract at will in order to see how these movements influence the tissue and the distance to which the signal can be propagated. “Our approach is based on a physiological response,” he details. “Because it is cells that are exerting the force, the signal remains within the realms of physiological reality.”
This work has notably focused on fibrosis, the hardening and loss of elasticity of a tissue that usually occurs following a lesion or inflammation. During this tissue change, fibroblasts are in a way reactivated and produce an excess of collagen. This reactivation drives the cells to exert even more pressure on their environment, thus inducing the activation of their direct neighbours, triggering a vicious circle and propagating the fibrosis to all the tissue.
Precursor mechanical forces
The way cells exert forces and influence one another is also central to the work of Jean-Léon Maître. A CNRS research professor at the Genetics and Developmental Biology unit3, he is studying the development of mammalian embryos, mainly in humans and mice. “Although the chemistry of cells is important, changes to the shape of an embryo mainly result from mechanical forces,” he explains. “These stimuli can also be transformed into a chemical response, which will then induce gene expression.”
Maître uses fluorescent markers to observe adherence between cells and how they exert their pressure by means of proteins that contract their surface. The scientist also employs micropipettes to measure the force required to deform the surface of cells, and laser-guided optical tweezers to explore the mechanism inside a cell.
“We were able to see that during the development of a mammalian embryo, the forces and cells involved change over time. The cells that pull the hardest are found inside the embryo. When the latter only comprises sixteen cells, it is their position that defines those that will remain inside the embryo and those that will form the placenta. This separation has long been studied by focusing on chemical criteria, but in fact it is mechanical forces that act upstream of changes to gene expression.”



Exertion of these forces starts inside the embryo from the eight-cell stage, just a few hours after fertilisation. Following asymmetric division, the most robust daughter cells manage to drag themselves inside the cellular mass, thus guaranteeing their participation in the embryo rather than the placenta. Embryogenesis continues with the formation of a fluid pouch within the embryo (the lumen) where hydrostatic forces fracture the contact points between the cells, allowing the fluid to accumulate where these adhere least to one another. Contractions around the cavity also shift the lumen, like air in a half-inflated balloon. “By modifying the adhesion and contractility of cells, it is possible to control where the embryo will attach itself,” underlines Maître. “These discoveries could help to improve fertility.”
A new approach: theramechanics
In terms of the therapeutic opportunities arising from mechanobiology, Yves Rémond is looking far into the future. This emeritus professor at the University of Strasbourg and ECPM European school of chemistry, polymers and materials, who is also a member of the ICube Laboratory4, has devoted his career to studying the mechanical properties of composite materials and polymers, to which he has gradually added biological materials. “The physical and mechanical principles remain the same whether the material is inert or living,” he notes. With Rachele Allena, lecturer at Université Côte d’Azur and member of the Jean-Alexandre Dieudonné Laboratory5, Rémond has developed the concept of theramechanics: therapies designed around the evolution of the mechanical properties of cells, tissues, bones and organs.
“Cancer cells spread notably thanks to two major and harmful actions of a mechanical type,” details Rémond. “They form metastases as they move, according to a speed and direction that change depending on the rigidity of the biological surfaces – such as tissues – they encounter. And when passing from one tissue to another, cancer cells need to find their way into highly confined spaces, so they must markedly change their shape.”
It is in this latter area that Rémond believes research can make a difference, since a confined and complex environment means extreme contortion for the cells. According to him, if it was possible to “make the nuclei of these cells more rigid, they could be prevented from spreading”, and consequently their propensity to metastasise be limited.


Rémond also thinks that mechanical properties should be included in work on biological digital twins. These model organs, specifically designed for each patient, are central to the development of personalised medicine. They could help to take better account of the history, age and specificities of individuals. These different options are still a long way from the stage of clinical trials, but mechanobiology is already laying their foundations. “So far, mechanobiology has mainly been studied in vitro, and it is necessary to develop in vivo approaches,” concludes Maître. “We still lack well adapted tools, but in the same way as biochemistry has revolutionised biology, our discipline will benefit from the emergence of scientists who are trained as pure biophysicists.” ♦
Explore more
Author
A graduate from the School of Journalism in Lille, Martin Koppe has worked for a number of publications including Dossiers d’archéologie, Science et Vie Junior and La Recherche, as well the website Maxisciences.com. He also holds degrees in art history, archaeometry, and epistemology.