You are here
The extraordinary fate of cells
Combining European technologies and knowledge to focus on a single object, the cell, is a unique opportunity. The aim is to look at the different stages in cell life, from its healthy form to its trajectory towards disease. “The idea is to join the forces of the entire scientific community, based on three major pillars: the study of cellular components, the production of disease models, and artificial intelligence,” explains Geneviève Almouzni, CNRS senior researcher at the Nuclear Dynamics laboratory.1 The year 2019 thus saw the afoundation of the European LifeTime consortium, which she coordinates. It brings together more than 100 research institutions and hospitals from 18 European countries,2 including the CNRS for France, and is receiving support from biotech companies and the pharmaceutical industry.
The reason why the 40,000 billion cells in our bodies are of so much interest to research scientists is that their lives follow a path that is all but straightforward. From the moment they come into being until they specialise to become liver, skin or brain cells, for example, these “building blocks” change their shape and function (a process called cell differentiation). They therefore live a life of perpetual change in the body, along trajectories which, if they deviate, may lead to disease: cancer, Alzheimer’s, cardiovascular conditions, etc.
The days of high-throughput sequencing
“We still know little about the overall functioning of cells. We hope to understand why a cell follows one pathway rather than another,” explains Almouzni. “And we can achieve this through the in-depth study of the detailed mechanisms that govern their lives,” adds Giacomo Cavalli, a senior researcher at the Institute of Human Genetics (IGH),3 in charge of the consortium for the CNRS. Central to these mechanisms is gene expression, a series of processes by which hereditary information, coded in the DNA of cell nuclei, is read in order to produce molecules in the body, one example being proteins. It is also necessary to closely study the interactions between cells and how they are influenced by their environment.
By mapping the fate of neural cells in the context of Alzheimer’s, for example, “we may in the future be able to detect a signal before the disease develops,” says Xosé Fernández, chief data officer at the Institut Curie. This would be a major step forward, because “when sufferers come to surgery, the cells in their brains are already so damaged that they have nearly reached the equivalent of a metastatic stage in cancer,” he points out. And even when the onset of symptoms enables the early diagnosis of chronic diseases, it is still not known why the tissues have deteriorated so much.
LifeTime has been set up because genomic and cellular analysis techniques are booming. Thirty years ago, the human genomeFermerThe genetic material of a species. project aimed to sequence all human genes within a decade. Today, new high-throughput sequencing technologies have made it possible to perform this operation in less than one day. “Back then, we thought that the human genome comprised 50,000 genes, but we now know that there are only 20,000,” adds Fernández. “High-throughput sequencing has given us access to the human reference genome” – a sort of “average” that combines the sequencing of several individuals and does not reflect personal diversities.
But even if it comes from a single subject, genetic data remains a standard resulting from the combination of several entirely different cells, each of which, although it contains the same initial genetic code as others, will have expressed a set of different proteins. This is due to the random nature of genetic expression as well as numerous other factors – some of them still unknown – which result in a different DNA reading and may activate certain genes and not others, etc.
This observation led to the development of single-cell sequencing techniques “which enable extraction and analysis of the genome of a particular cell and can confer each one with an individual characterisation,” explains Fernández. So it is now possible to study the genes expressed or the proteins produced by each cell, and to understand the differences in dynamics between a cancer cell (following a genetic mutation) and a healthy one, for example. “That is the very core of LifeTime,” he emphasises.
Significant progress has also been achieved in the field of microscopy. “Before the 2000s, the resolution of fluorescence microscopy was limited to 250 nanometres,4 while the double-helix structure of DNA is only 2 nanometres wide,” Cavalli points out. The length of proteins, RNA or oncogenic complexesFermerGenetic sequence that causes the formation of a cancer cell once it has been transformed by a virus or another agent. is also comprised between 1 and 10 nanometres. These former methods did not allow for visualising protein complexes or nucleic acid structures smaller than the limit of resolution. Yet the latter has considerably improved over the past decade, making it possible to distinguish biological structures of only a few nanometres.
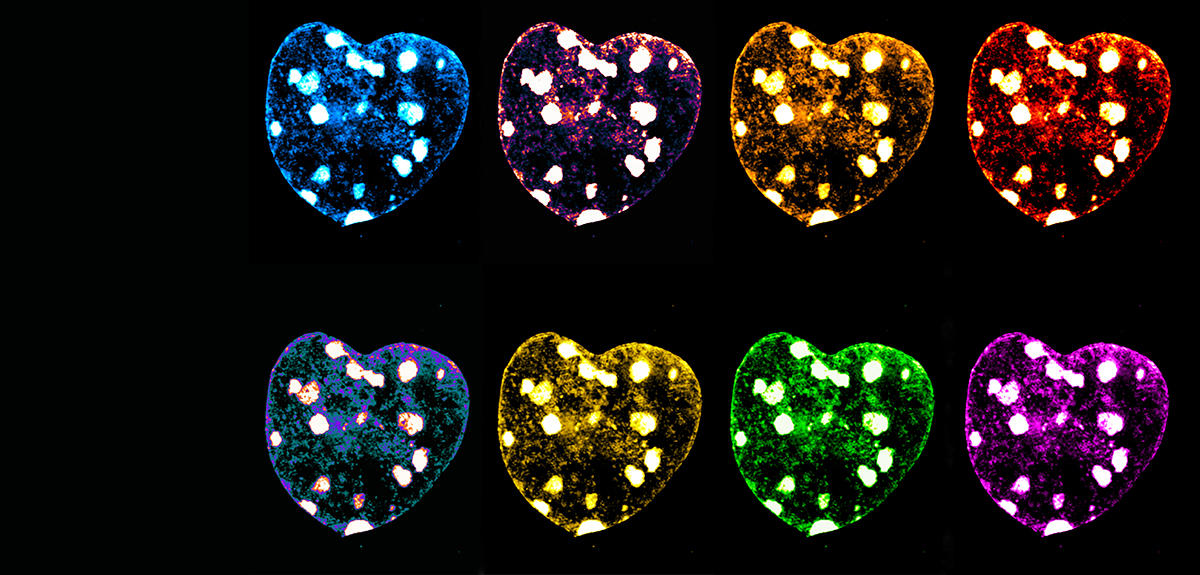
And there is more: new cutting-edge fluorescence techniques have now matured, explains Marcelo Nollmann, senior researcher at the Centre for Structural Biology (CBS).5 “Until now, we only had four or five colours to label the objects present in a cell. It was thus very difficult to picture something like the structure of a chromosome with only four shades to choose from.”
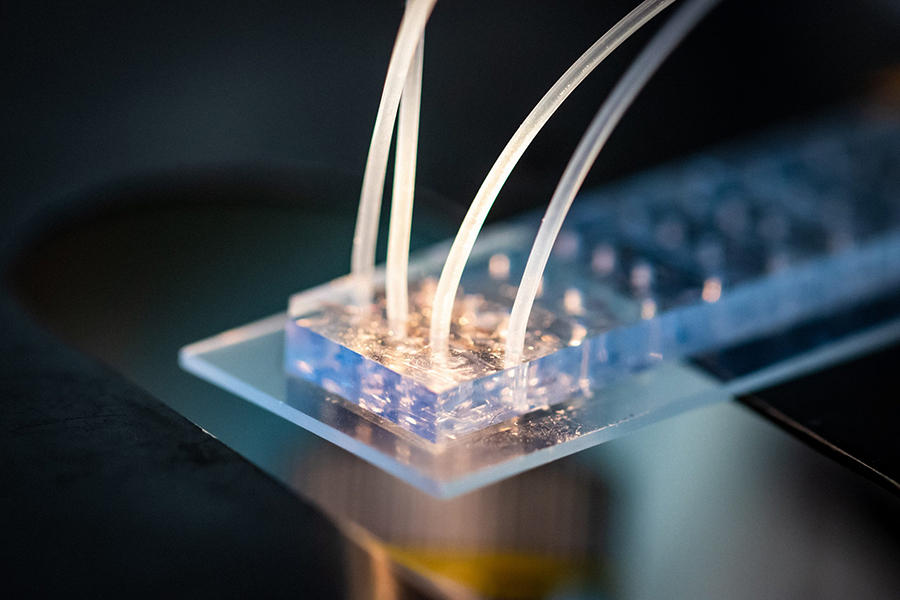
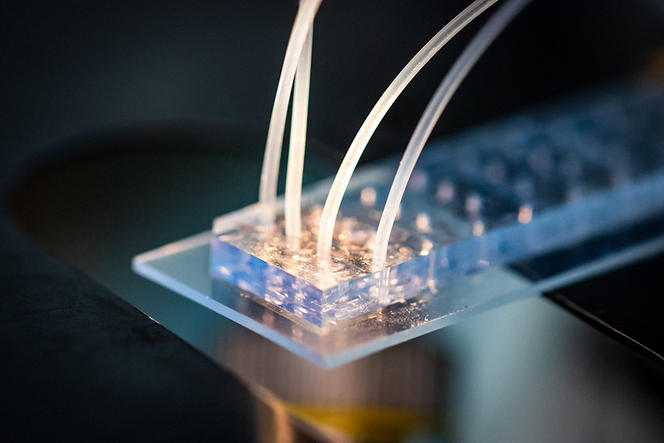
Today, a combination of microfluidic technologies (the manipulation of fluids at micrometric scales) and fluorescence microscopy have enabled the superimposition of signals in order to have dozens of colours at the same time.
An ID for cells
“We can now visualise 50 to 500 DNA sites simultaneously and in the same cell, as well as hundreds of RNA species. This new approach enables us to trace the path followed by DNA in each cell under nanometric resolution,” Nollmann enthuses. Today, scientists can therefore detect which genes are expressed by an individual cell and how their expression is controlled by the three-dimensional architecture of genetic material (the 3D structure of the genome). “Based on these two elements, we can draw up an identity card for each cell, and determine whether it is normal or pathological,” he adds.
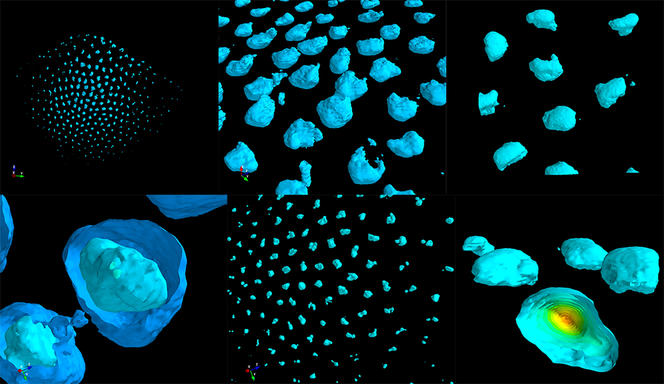


With his team, the scientist is now developing and applying these new imaging methods. The aim is to study changes to the 3D structure of both the genome and the transcriptional programmeFermerA process that orchestrates for each cell how the transcription of thousands of DNA segments in the genome is regulated in order to produce certain types of RNA molecule necessary to maintain the identity and biological functions of the cell. of different cells during the course of a disease such as diabetes. “Before, we’d take a whole pancreas, grind it and analyse the average genetic expression of all the cells.” However, this organ contains different cell types: beta cells that produce insulin, or alpha cells that make glucagon. “Thanks to the new microscopy techniques, we are starting to understand how these different types are organised spatially within a tissue or organ, and how they change as a disease progresses. In particular, visualising the genomic structure in each cell type will enable us to decipher the gene expression regulation mechanisms at play as the condition evolves,” explains Nollmann.
Models for all diseases
This is the whole point: mapping the paths of cells and how their gene or protein expression changes, in order to propose a model that can be applied to all conditions and be used by research laboratories throughout Europe. This will enable the pooling not only of knowledge and technologies, but also of biological samples, as some countries’ biobanks are much larger than ours. “For example, in Israel, there is a tradition of taking far more blood samples. As a result, the country has a very large biochemical databank covering several years, which could serve to develop models for non-pathological cells,” suggests Fernández.
The data collected from across Europe “will be made available to all consortium members so that no-one is working in isolation. In this way, the scientific community will gain time by sharing the most effective methods,” Almouzni predicts. The longitudinal monitoring of single cells will be supplemented by studies on organoids; these are 3D cell cultures that imitate the functioning of an organ; for example, some organoids are already being used to mimic colon cancer. The idea is to harvest colon cells from a patient and differentiate them, or in other words return them to the state of a stem cellFermerAn undifferentiated cell that is able for example to generate specialised cells (liver, heart, skin, etc.) through differentiation.. “After that, we know how to re-differentiate these cells in order to create a mini-intestine that is available for study,” she explains.



The development of these organoids offers considerable hope to all the laboratories that will have access to them, and particularly that of being able to study in culture the efficacy of numerous treatments “or the influence of pathogenic agents, in order to understand how they alter the organoids or lead to the formation of cancers”. The aim is to share a battery of biological tissues “on which we can screen thousands of molecules and see if they are effective”, hopes Cavalli.
Better targeted treatments?
The scientist is also focusing on applications in neurobiology. Although numerous genomic studies have been performed on pathologies involving cognitive dysfunction, such as autism, the role of the genes involved in these conditions remains little known. “Cells collected from the blood of patients with neurodevelopmental disorders can be deprogrammed, returned to the state of stem cells and finally reprogrammed to become neurons.” Without having to take samples in the brain, the scientists can obtain neurons containing the genetic code of the patient and study them at the scale of a single cell. “We have realised that certain neurological functions are disturbed in these patients. Had we been limited to analysing the genes, this observation would have been impossible,” Cavalli adds. “This offers new opportunities to find both explanations and therapies.”
The members of the European consortium also hope that zooming in on the machinery of a cell will enable them to understand why certain tumour cells respond to treatments and others do not. The scientists already have tools that can mimic metabolic dysfunctions and the tumour process. “We can use these tools to elucidate the impact of cancer on the cell and perhaps intervene at the earliest possible stage,” he suggests. The prevention of cardiovascular disease is also being targeted. Indeed, after an infarction, the RNA analysis of cardiac cells might reveal molecular signals of recovery from the after-effects of a heart attack, or even a deterioration of the patient’s state!
Towards personalised therapies
LifeTime also intends to involve the pharmaceutical industry and give it cues for understanding the life of cells. Such collaborative effort will enable sharing of knowledge and tools and contribute to the more efficient development of personalised therapeutic solutions; indeed, to discover drugs, pharmaceutical research often needs to test thousands of molecules and see which ones work or not, “without really understanding why”, notes Cavalli.
AI to the rescue
Analyses of DNA, proteins, RNA, metabolic processes or the cell environment generate masses of data. The genetic screeningFermerIn pharmacology or genomics, techniques designed to study and identify biologically active molecules with novel properties from among those in a databank. of just 10,000 cells from the human body “already requires huge computing capacity”, stresses Fernández. Hence the third main pillar of LifeTime: artificial intelligence (AI). While specialists in the life sciences will harvest data, bioinformatics experts will work on processing it so that, for example, they can reveal genetic variants or early signals of disease. “By obtaining a molecular map of the course of a disease, artificial intelligence may be able to produce a model for its detection,” Fernández hopes.
Another example is to use the medical history of patients, he explains. “Cancer is generally a condition related to ageing. Our patients are 66 years old on average, and they have often taken treatments throughout their lives for associated ailments. Machine learningFermerAn artificial intelligence technique where the machine learns from data in the absence of any logical rules dictated by the human programmer. can cross-reference this drug data in order to determine whether past therapies might potentiate, or on the contrary annihilate, a cancer treatment.”
LifeTime will adopt an ethical and societal approach to science, combining technological, fundamental, industrial and civic features. “We are paying particular attention to personal data protection,” emphasises Almouzni. “That’s the advantage of a European consortium,” echoes Fernández. Indeed, Europe implemented its General Data Protection Regulation (GDPR) in 2016, unlike the US, for example. “The aim is to build up a federated European data system, like the Health Data Hub set up in France in 2019.”
The next step is to secure long-term European funding. In early September, the members of LifeTime published an article in the journal Nature6 to outline the project’s main prospects. The signatories to this article “were not able to give credit to all the scientists who contributed to it”, regrets Almouzni, thus underlining the importance of collective effort in this decisive research. “We have reached a turning point in the study of cells – a dream that is becoming reality,” she enthuses.
- 1. CNRS / Institut Curie / Sorbonne Université.
- 2. https://lifetime-initiative.eu/
- 3. CNRS / Université de Montpellier.
- 4. A nanometre is equal to a millionth of a millimeter.
- 5. CNRS / Inserm / Université de Montpellier.
- 6. “LifeTime and improving European healthcare through cell-based interceptive medicine”, N. Rajewsky, G. Almouzni, G., Gorski et al., Nature, September 2020.
Explore more
Author
Léa Galanopoulo has a biology degree and is currently studying scientific journalism at Paris-Diderot university.