You are here
Virology: Between a Marathon and a Race against Time
Sometimes qualified as biological objects, viruses lie at the frontier of the living world. They nevertheless have an extremely strong impact on living organisms, whether plants or animals. So what differentiates them from other microscopic creatures such as bacteria, protozoans or fungi? “Viruses are absolute parasites; they have no choice but to enter a cell in order to replicate,” explains Yves Gaudin, CNRS research professor and leader of a team in the Virology Department at the Institute for Integrative Biology of the Cell (I2BC).1 “They are strictly dependent on the metabolism of this host.” Unable to synthesise their own proteins, viruses are forced to divert the internal machinery of cells to their own benefit. Virologists study these parasites that can mutate and cross the barriers between species, focusing in particular on those that are the most dangerous at a given time, but they also have the difficult task of trying to anticipate future epidemics. So which strategies do they adopt?
First of all, it is necessary to understand that viruses are studied at different scales: molecular, cellular, in the body and, in the event of an epidemic, in an ecosystem or entire society. At the molecular level, virologists determine the structure of the virus and its proteins. Indeed, viral proteins are the therapeutic targets that need to be blocked to prevent the pathogen from penetrating the cells where it can replicate. This work uses structural biology tools such as nuclear magnetic resonance (NMR) or electron cryomicroscopy (cryo-EM), a technology whose recent advances have considerably facilitated the work of virologists and microbiologists.



At the cellular level, the scientists focus on the interactions between a host and the parasite that is trying to redirect its internal machinery. “The cells also have their own immune system, which enables them to know whether they are being infected,” explains Yves Gaudin. “They then produce antiviral proteins: interferons and restriction factors. This is a very important area of research in the context of the current crisis.” Indeed, viruses whose genome is made up of RNA, like HIV, rabies, influenza and SARS-CoV-2 (which is causing the current epidemic) can block these cellular responses.
From the molecule to society
In the body, viruses and infected cells are not isolated. The pathophysiological approach takes account of the presence of macrophages and lymphocytes, as well as of the whole body that is infected. This is an environment that is in principle harmful to the virus, but can also turn against the patient. “SARS-CoV-2 seriously disrupts the immune system,” emphasises Yves Gaudin. “It can cause what we call a cytokine storm.” When this happens, the proteins (cytokines) responsible for marking the targets for the immune system are released in such quantities that they cause generalised inflammation, which is fatal if it remains untreated. This phenomenon is observed with other RNA viruses such as dengue fever, and is suspected to have been partly responsible for the extremely high death rate observed with Spanish flu.
Finally, epidemiology examines and models the spread of a virus at the population scale. This starts from the moment it crosses the species barrier until political measures are introduced to halt and cure the disease. At this level, the human sciences may be introduced in support.
However, virology does not just focus on pathogenic agents. Most viruses are harmless to us, but it is no less important to study them. “We consider viruses to be harmful, but they have always coexisted with cells and participated in the evolution of life on Earth,” explains Chantal Abergel, CNRS research professor and head of the IGS.2 “Without them, there would be no mammals! It is indeed a protein of viral origin that allowed our ancestors to develop a placenta so that they would no longer need to lay eggs.”
Giant viruses
Research on viruses sometimes has surprising results. Chantal Abergel and her colleague Jean-Michel Claverie participated in the discovery of one of the first known giant viruses, which had remained frozen for 30,000 years in the Siberian permafrost. Such pathogens had previously escaped the attention of scientists because of the systematic filtering of samples that does not allow them to pass. Thus, while influenza possesses around ten genes, up to 2500 have been identified in pandoraviruses. They may also be able to create their own genes, independently of evolution. “Viruses may represent an abandoned metabolic pathway,” Chantal Abergel adds. “It is as if they had lost their fight with the cellular world and parasitism had become their only solution.”

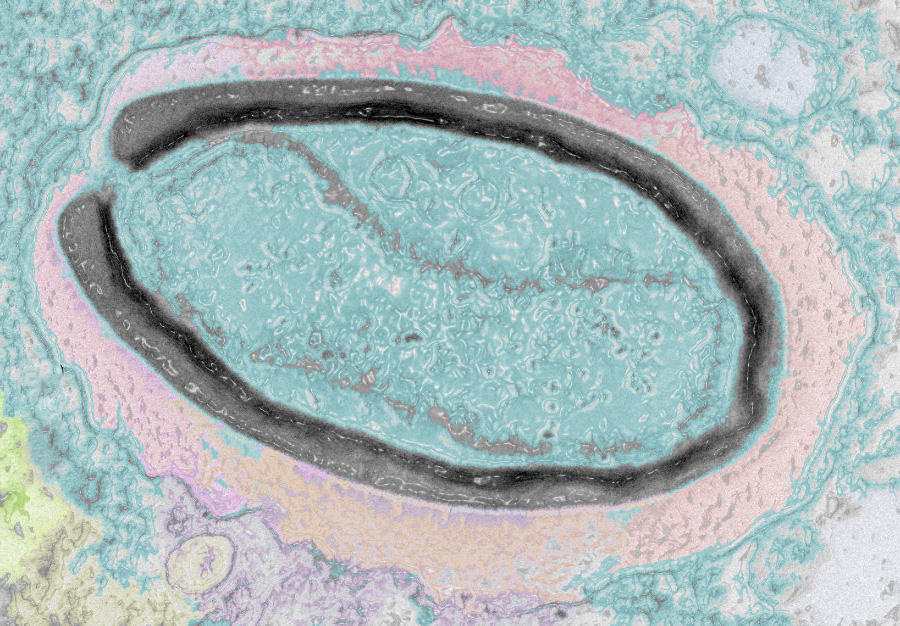
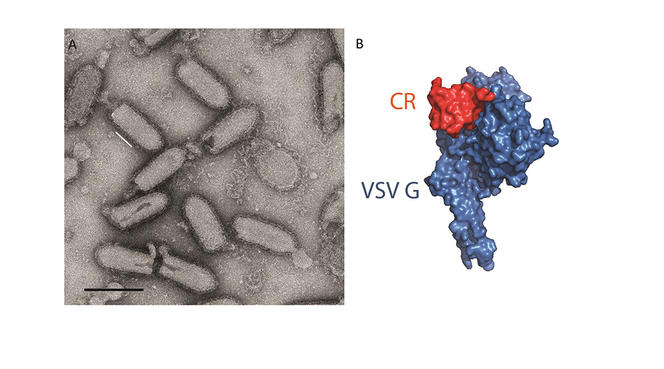
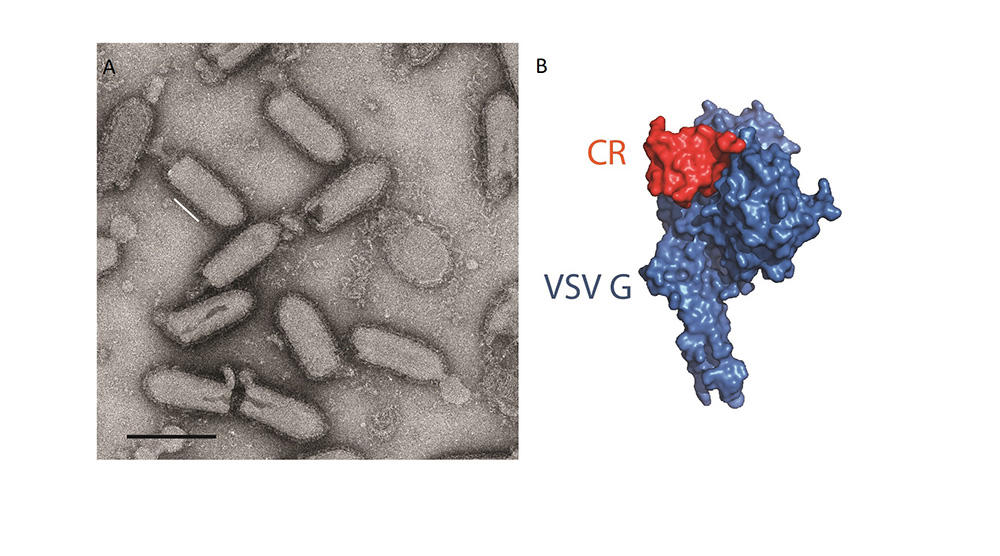
Of additional interest to research is the ability of viruses to very precisely target certain cells that can be redirected for use in gene therapies. For example, Yves Gaudin uses the vesicular stomatitis rhabdovirus (VSV)3 to attack cancer cells. Other viruses, referred to as bacteriophages, only target bacteria; they are not dangerous to humans and could serve to eliminate bacteria that have become antibiotic-resistant. Some viral enzymes, such as reverse transcriptase, are now standard tools in molecular biology. And finally, scientists use viruses to investigate cell functions, observing the interactions they develop within infected cells to gain a better understanding of certain machineries and signalling pathways in the host.

Despite these interesting perspectives and the importance of being able to fight epidemics, many virologists consider that their discipline has not received sufficient support during recent decades. Although scientists defending their own interests or being forced to doom-monger is nothing new, the scale of the Covid-19 crisis has certainly proved them right.
“We lack specific agencies dedicated to emerging viruses,” regrets Bruno Canard, CNRS research professor and deputy director of the AFMB laboratory.4 “Numerous studies have been performed on their sequencing, but we need to understand the mechanisms that govern their entry into cells, their replication and their interactions with the immune system. Too few teams are working on these topics. Electron cryomicroscopy has given rise to a real revolution in structural biology, but we are still seriously under-equipped in France.”
Bruno Canard specialises in viruses with an RNA genome, a family that causes Ebola, chikungunya and dengue – and also includes coronaviruses. These infective agents generally have a reduced genome, and because RNA is less stable than DNA, they mutate and evolve more rapidly. Some of these errors in the gene copy may hamper the functioning of the virus, but SARS-CoV-2 contains an enzyme, exonuclease, which repairs the most important defects.
Solutions that must remain effective
“Unlike the proteins present on the viral envelope that are constantly fighting the immune system, the RNA synthesis apparatus of the virus changes little,” suggests Bruno Canard. “Indeed, it is more subject to the laws of thermodynamics and kinetics, so its limited margins for manoeuvre make it an ideal target.” Using drugs to block this apparatus is indeed the strategy that has been successfully deployed in antiviral therapies for HIV, hepatitis C or herpes.
Viral genetic variations are a problem addressed by Roland Marquet, CNRS research professor in the ARN laboratory.5 He studies HIV and the influenza virus, whose particularity is to be segmented into eight parts that can be assimilated to viral chromosomes. Now, if two different influenza pathogens infect the same cell, they may reassort their genetic material and replicate a hybrid virus. This phenomenon enables them to evolve extremely rapidly and generate viruses with considerable pandemic potential. Understanding these genetic reassortment mechanisms is therefore an important challenge.
“Virological research must remain as broad as possible,” insists Roland Marquet. “We cannot predict which viruses will pass from animals to humans. The pandemics of the 20th and 21st centuries were all caused by influenza viruses or, in the case of AIDS, by a retrovirus. If we limit our work to those we think are the most important, we will be powerless when we come under attack from a coronavirus.” Chantal Abergel is of the same opinion: “We absolutely need to understand the physiology of all viruses. SARS-CoV-2 is not the most infectious or the most fatal, and I do not know how we would be able to react if a new and much more dangerous virus were to appear.”
In the context of the current pandemic, the French National Research Agency (ANR) has initiated a procedure to support 86 projects on SARS-CoV-2. From the Molecular Microbiology and Structural Biochemistry (MMSB) laboratory,6 Lauriane Lecoq is coordinating one of them; using nuclear magnetic resonance, she is observing the interactions between different molecules and the ORF8 protein of the virus. This information is crucial for steering the search for drugs. Lauriane Lecoq is already applying these methods to the hepatitis B, hepatitis D and dengue viruses, focusing in particular on capsid proteins. The latter assemble to form a protective and sometimes spherical layer around the DNA or RNA of the virus. When a cell is contaminated, the capsid and its envelope penetrate it and the capsid releases infective agents into the nucleus. This protein is therefore a key pharmaceutical target.
“Fundamental research in virology needs support,” insists Lauriane Lecoq. “Decisions on where the money should go should not just be taken in the light of current events; it is necessary to look ten to twenty years into the future. Even viruses for which there is a vaccine or effective treatment, such as hepatitis B and C, could mutate.” All this contributes to the paradox of a multi-faceted and essential discipline that often comes to mind only in an emergency.
- 1. CNRS / CEA / Université Paris-Saclay.
- 2. Laboratoire Information Génomique et Structurale (CNRS / Aix-Marseille Université).
- 3. The rabies virus is the best known type of rhabdovirus.
- 4. Laboratoire Architecture et Fonction des Macromolécules Biologiques (CNRS / Aix-Marseille Université).
- 5. Laboratoire Architecture et Réactivité de l’ARN (CNRS).
- 6. CNRS / Université Claude Bernard Lyon 1.
Explore more
Author
A graduate from the School of Journalism in Lille, Martin Koppe has worked for a number of publications including Dossiers d’archéologie, Science et Vie Junior and La Recherche, as well the website Maxisciences.com. He also holds degrees in art history, archaeometry, and epistemology.