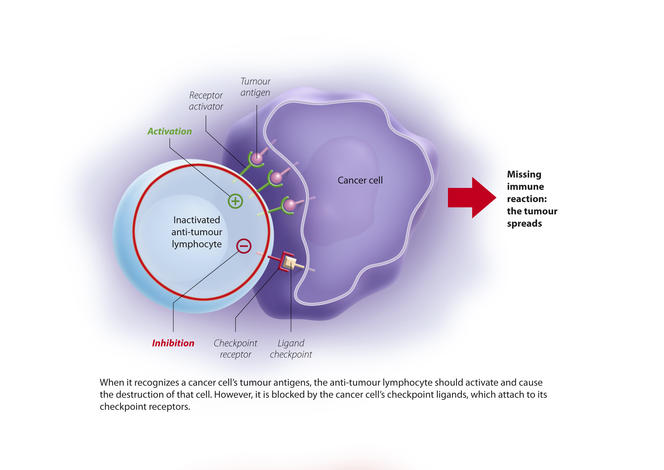
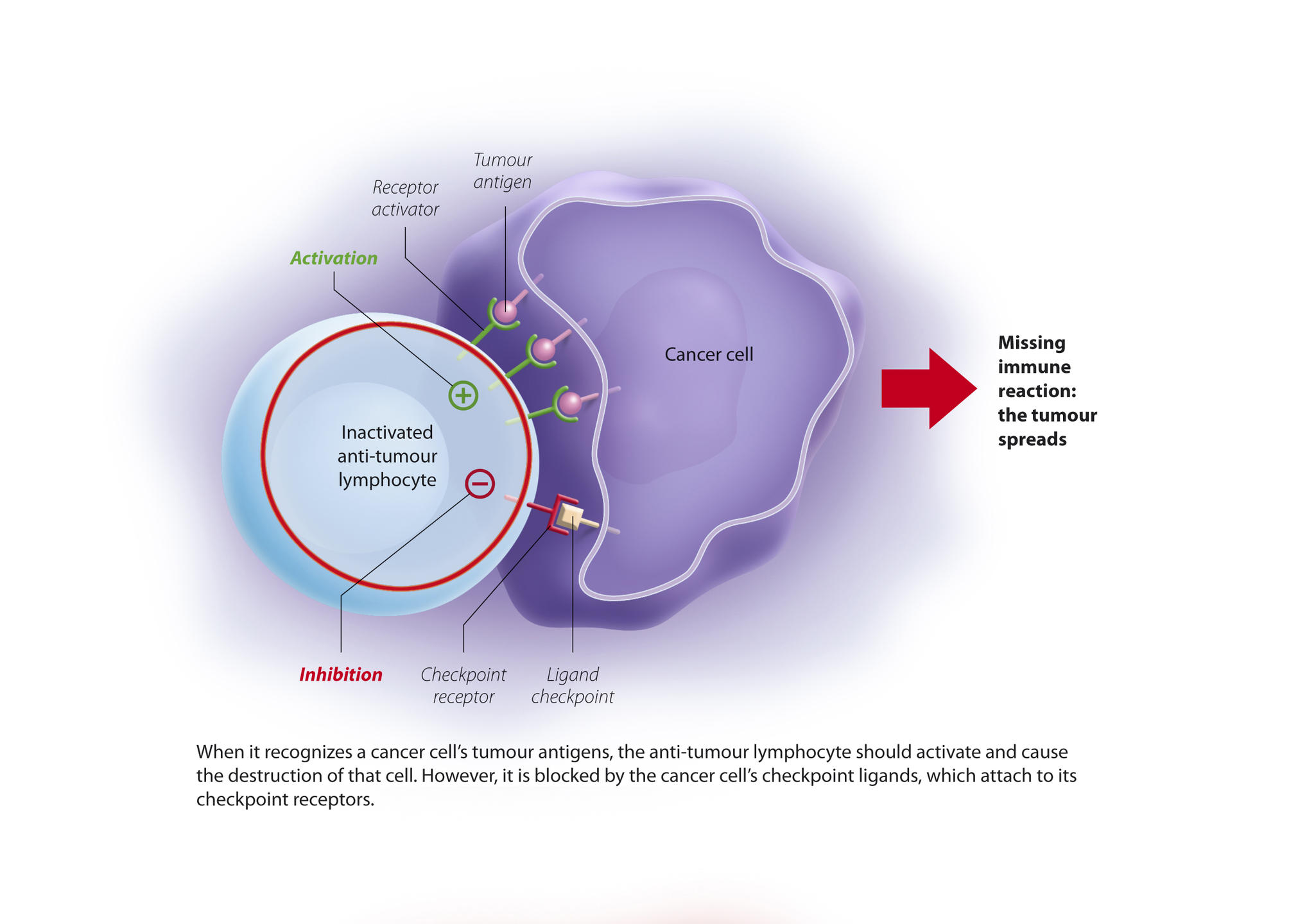
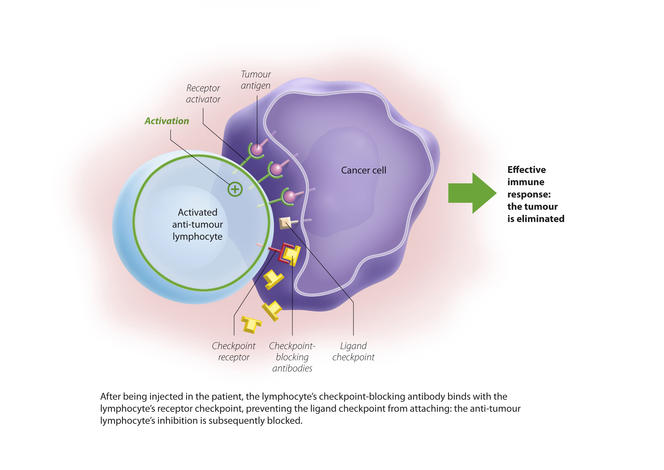
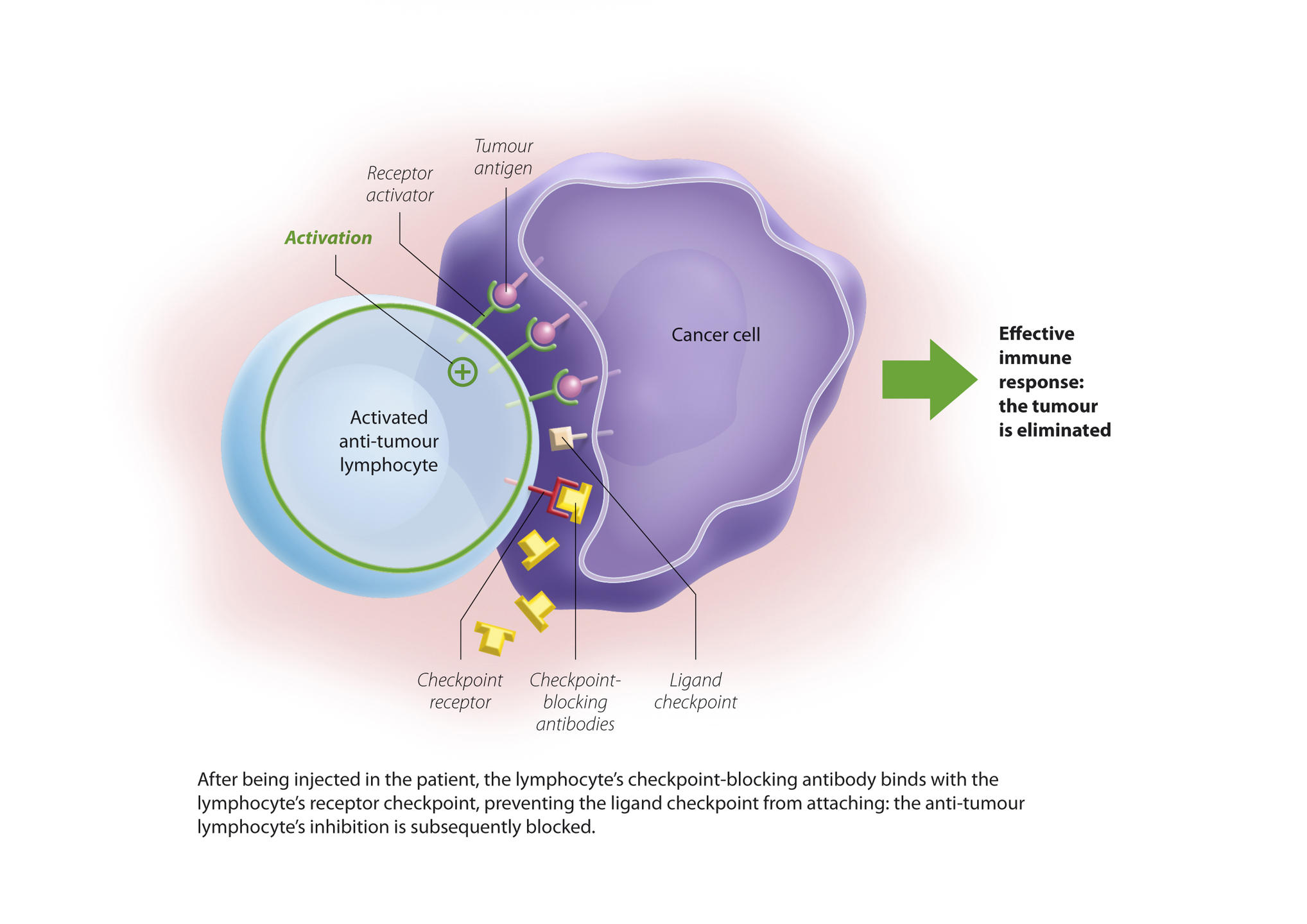
You are here
Cancer: the Immunotherapy Revolution
In October 2018, the Nobel Prize in Physiology or Medicine was awarded to Tasuku Honjo and James P. Allison who, in the mid-1990s, had set out to demonstrate the possibility of using the immune system to destroy tumours. Twenty years later, immunotherapy is starting to transform the management of some cancers that were previously incurable.
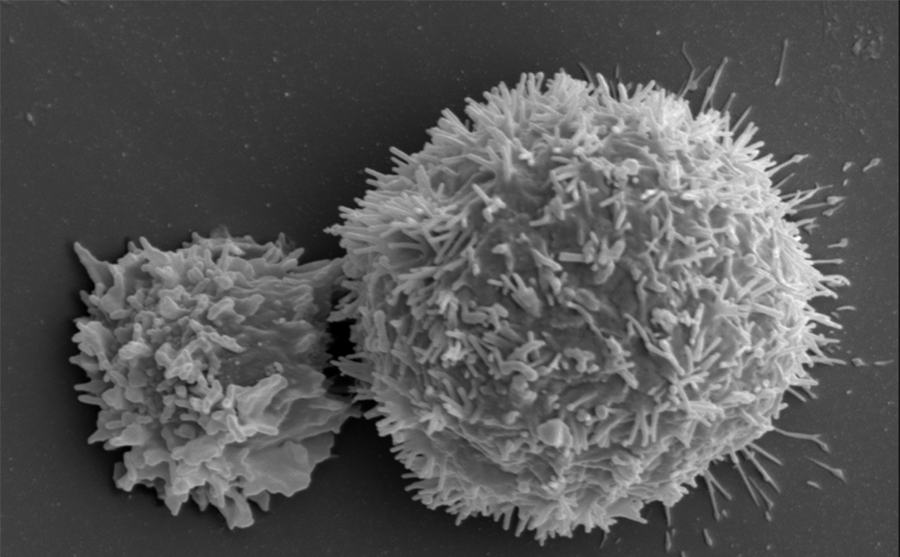
The immune system, composed of cells produced by the bone marrow such as lymphocytes, is usually programmed to eradicate the abnormal proliferation of infectious agents or mutated cells. But its action on cancer cells is often too weak, or too slow, to stop the progression of the disease and trigger the destructive action of lymphocytes.
In light of this, one therapeutic option since the 1970s thus consisted in stimulating the immune system to target cancer cells. Attempts to inject cytokines – molecules that are able to trigger an immune reaction – or immunostimulant tuberculosis vaccine proved little successful, as the immunotherapy was not specifically directed against the disease.
Checkpoints: the crucial target
Discovery of the first tumour antigens changed the situation and opened the way towards much better targeted techniques. Indeed, tumour cells express antigens on their surface that are not found in normal cells. These antigens therefore make good targets for a potential "cancer vaccine”. In parallel – and this was the subject of the discoveries rewarded by the Nobel Prize – scientists demonstrated that when faced with a cancer cell, the action of lymphocytes is naturally inhibited by molecular brakes, which are activated by immunological control points, or “checkpoints.”
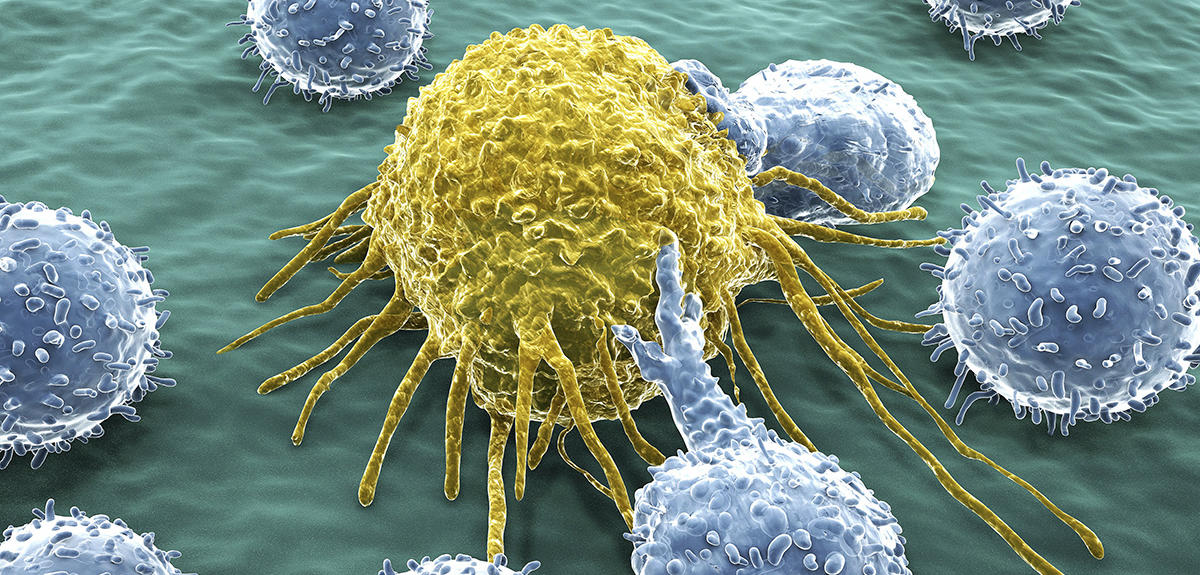
Two checkpoints were initially identified: PD-1 and CTLA4. PD-1 is expressed by lymphocytes and binds to its ligand (PDL-1) which is expressed by cancer cells. The immune system is then blocked and no longer recognises the tumour. The idea is therefore to release these brakes to reactivate the immune response and destroy the malignancy. These findings have enabled significant therapeutic advances through the development of checkpoint inhibitors, i.e. antibodies directed against these control points and able to block them.
In 2010, an anti-CTLA4, ipilumumab, was thus put on the market to cure advanced melanomas that are usually highly resistant. And anti-PD1s such as nivolumab generate a sustained response in one-third of patients with skin melanomas or lung cancers.
The paradox of immunotherapy
"Immunotherapy has improved considerably. New approaches consist in releasing the brakes. Each time a new checkpoint is identified, it is now possible to create an antibody to neutralise it," explains Jean-Jacques Fournié, CNRS senior researcher and head of the “lymphoma immunotherapy” team at the CRCT1. It is estimated that one in three patients may respond positively to the immunotherapy currently available. Research therefore seeks to improve understanding of the various immunotherapy response criteria.
The first condition for a good response is that the patient’s tumour expresses checkpoints that can be neutralised by the antibody. “It seems obvious but in fact this was controversial," Jean-Jacques Fournié explains. "Indeed, these biomarkers are not always stable over time; they may be expressed temporarily for a few days, and then disappear. Not to mention the fact that their quantification is not necessarily standardised.” Today there is nevertheless general consensus that the expression of PD-1 is a criterion for good response to immunotherapy.
The second prerequisite, which alone reflects the entire paradox of immunotherapy, is that the more a tumour contains mutations, the better it will respond to the antibodies. “This is paradoxical, but can easily be explained from an immunological point of view: the more mutations, the more neo-antigens and thus potential targets for treatment,” explains Professor Fournié. This is the case for certain lung cancers (particularly in heavy smokers), highly mutated colon cancers or hereditary cancers such as BRCA1 breast cancer, whose prognosis was previously very poor.
Other characteristics also point to a good response to immunotherapy, such as a tumour located in a tissue that is naturally well infiltrated with lymphocytes. This is the case for lung cancers (again) as well as renal, skin and colon malignancies or certain lymphomas. Conversely, a "cold", little inflamed tumour (cancers of the brain, eye, testicles or bone marrow) will be synonymous with a poorer response to immunotherapy. “One research avenue to improve the efficacy of these immunotherapies would be to irradiate these cold tumours, thus causing an inflammatory effect and giving the immune system access to cells that were previously unattainable,” explains Jean-Jacques Fournié.
Identifying and releasing the brakes
Responses to immunotherapy at present are therefore heterogeneous. “We are all agreed that it would be naive to think that we can treat the disease with just two checkpoints,” the scientist says. In addition to PD-1 and CTLA4, there are in fact around fifty points that hinder the immune reaction in the event of cancer, “and the more the collection of brakes is complete and combined, the better the cancer develops,” adds Jean-Jacques Fournié. For treatment to be as effective as possible in the future, the idea is to target several checkpoints at the same time, in the same way as a broad-spectrum antibiotic.
With this in mind, Jean-Jacques Fournié, Don-Marc Franchini and their team started their research work in 2013 with the aim of understanding how, once activated, lymphocytes regulate the expression of these checkpoints. They realised that the latter are controlled by the same family of molecules, microtubule inhibitors, which are already used in some chemotherapy regimens, e.g. trastuzumab. By inhibiting these microtubules, the checkpoints are no longer expressed and the immune system can come into play.
“We saw that lymphocytes control the expression of checkpoints at the level of RNA. And this transfer of RNA is associated with the production of small molecules called stress granules,” points out Jean-Jacques Fournié. This discovery is important because this mechanism is common to all checkpoint inhibitors, and does not just apply to PD-1! ‘”This is a breakthrough because if we could block these stress granules, we could also block all the checkpoints they carry!.” The team is now set on finding therapeutic leads inspired by this research on microtubules and stress granules.
A two-in-one antibody to release immunity
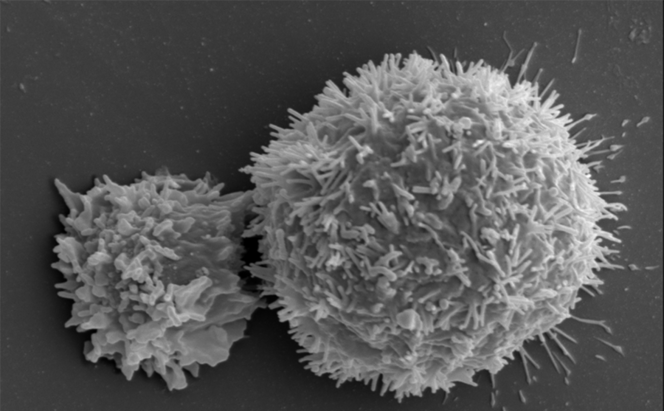
Work in the field of immunotherapy is focusing on both the discovery of new checkpoints and the anti-tumour potential of certain immune cells. This is the subject of work by Eric Vivier, Professor of Immunology at Aix-Marseille Université and a researcher at the CIML.2 Ten years ago, he and his team discovered a new type of immune cell: innate lymphoid cells (ILC). This is a large sub-group that includes natural killer cells (NK). “For many years, we were unaware of an entire section of the immune system. ILC are neither T-cells nor B-cells, but a completely new line that must now be included in all publications on immunology. And they are of course an additional lead for immunotherapy,” the scientist explains.
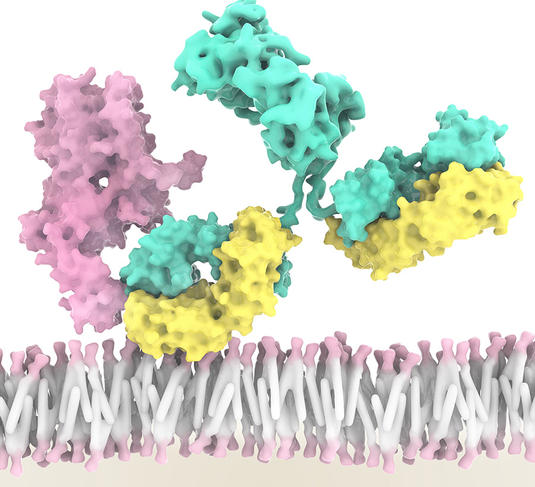
Five types of ILC have now been identified, each playing a role in inflammation and immunity. For example, NK cells can recognise and kill cancer cells, while at the same time driving the anti-tumour immune response of other immune cells. “An international team led by Innate-Pharma has developed an anti-NKG2A antibody called monalizumab. This new checkpoint inhibitor is able to stimulate the anti-tumour activity of both NK cells and subpopulations of T lymphocytes,” Éric Vivier stresses. As a result, the action of NK cells and certain T lymphocytes is no longer blocked. This is a triple performance as monalizumab can also boost the effect of durvalumab (an anti-PDL1) and of cetuximab, an antibody that recognises tumour cells.
What about adverse effects?
“When combining one or other of these two antibodies with monalizumab, we did not observe any additional adverse effects, which is really positive! Most of these effects are linked to the release of T lymphocytes, but not to that of NK cells, which are able to differentiate good cells from bad,” says Eric Vivier. This is all the more important as one of the crucial challenges of immunotherapy is still to limit the side effects of treatment.

Because it reactivates the immune system, immunotherapy can cause autoimmune diseases such as multiple sclerosis, diabetes or lupus. “By analysing pharmacology data at the global scale, we have found that patients receiving immunotherapy with checkpoint inhibitors experience ten times more autoimmune effects than other types of side effects. This is also a sign that it is working,” adds Jean-Jacques Fournié.
“All sparks will light a fire”
Like his colleague, Eric Vivier believes that the future of immunotherapy will involve a broad-spectrum strategy: “we are living through a revolution in cancer treatment. The idea is to add as many pillars as possible by accumulating checkpoint inhibitors, radiotherapy and an analysis of the tumour environment in order to find out how in parallel we can make this environment more immunostimulating. “All these sparks will eventually light a fire,” he adds.
The future of immunotherapy is promising and has aroused tremendous hope for thousands of patients with cancer. Each year, 400,000 new cases are detected in France. The challenge is to make this therapy accessible to all; the cost of a course of treatment with a monoclonal antibody can often reach €130,000. “We must bear in mind that all these advances are the result of basic research. Twenty years ago, when we started studying immune cells, our aim was not to treat cancer, and yet our work is now directly connected to the disease,” Eric Vivier emphasises.
Explore more
Author
Léa Galanopoulo has a biology degree and is currently studying scientific journalism at Paris-Diderot university.