You are here
Jean-Marie Tarascon, energy prodigy

"I wanted to be a farmer, and I was passionate about rugby." The 69-year-old Tarascon ultimately became a leading chemist (and major enthusiast of football). We partly have him and his teams to thank for the remarkable performance of the batteries in our computers, mobile phones, and electric vehicles. The co-signer of one hundred patents, and the author of more than 700 scientific articles, he has won twenty prestigious prizes, now including the 2022 CNRS Gold Medal.
From the French countryside to the American dream
As a teenager in Marmande, Tarascon imagined his future in the fields, like his father. Studies hardly interested him: the land took precedence over any curriculum. But he had five brothers, and not all of them could succeed their father managing the family farm. "I had to find something to do," he recounts with a lively accent from South West France, which he has never lost. "In secondary school I was fairly good in physics and chemistry, albeit without being captivated by the discipline. So after my baccalauréat degree I enrolled at the École supérieure de chimie de Bordeaux, where I met a passionate teacher who explained how the machines that surrounded us worked. I immediately found this fascinating, and for the first time I went to the library for hours to gather information on my own." The seed was planted, and would grow steadily.
At the École supérieure de chimie de Bordeaux, Tarascon met Régine, who would become his wife, and began to taken an interest in solid-state chemistry. He even made it his thesis topic at l'université de Bordeaux, before exporting his talent and the passion that drives it to the other side of the Atlantic, where the prestigious Cornell University hosted him for his postdoctoral fellowship. "I had practically never left my region, I had never been to Paris, so when I set foot there it was a shock: I felt lost, the weather was miserable." However, Tarascon quickly discovered the advantages of exile: "Conducting research in the United States at the time was extraordinary. One could feel the passion and competitive spirit. I felt totally free to conduct my experiments whenever and however I liked."
After his postdoc, the American dream continued, as he was immediately recruited by Bell Labs.1 The year was 1981, and mobile phones were still in the prototype phase (the first ones were sold by Motorola in 1984), although portable devices were already developing at lightning speed. The same was true of their indispensable component, batteries. A revolution had just occurred in this field in the form of incredibly efficient lithium-ion accumulators (see boxed text below). "Bell Labs had the world’s densest concentration of Nobel Prize winners per square foot, and they were all very accessible. All of the conditions were present to become a good researcher," he recalls.
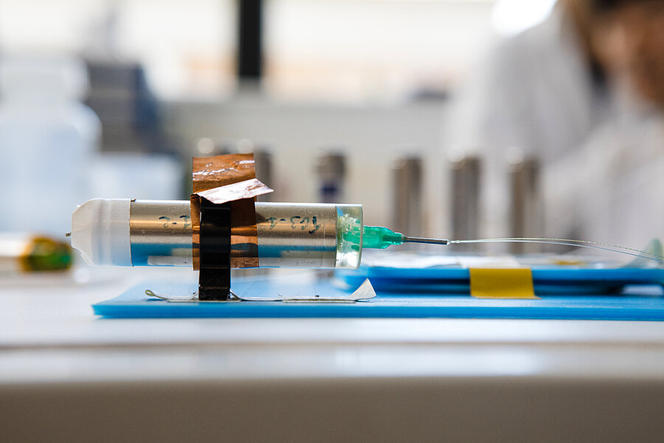

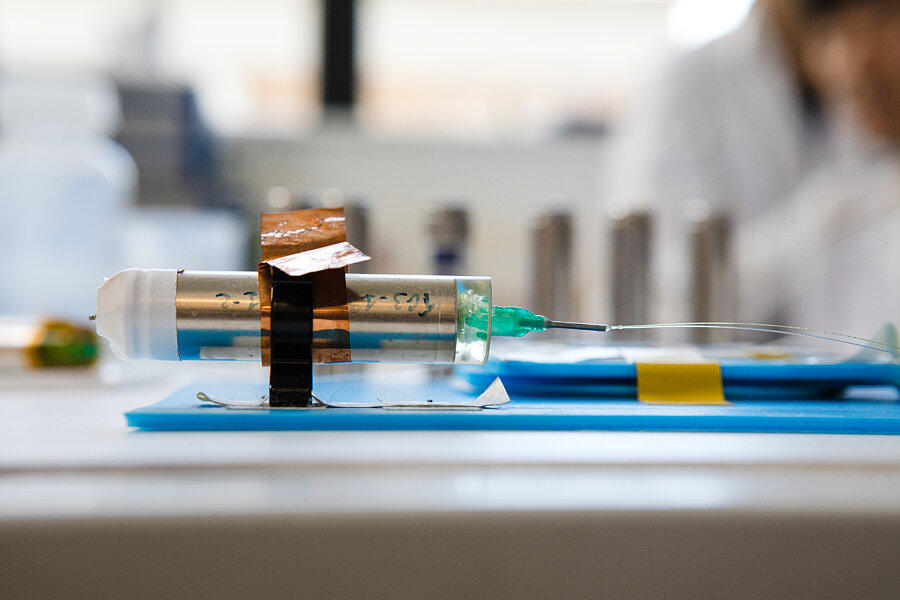
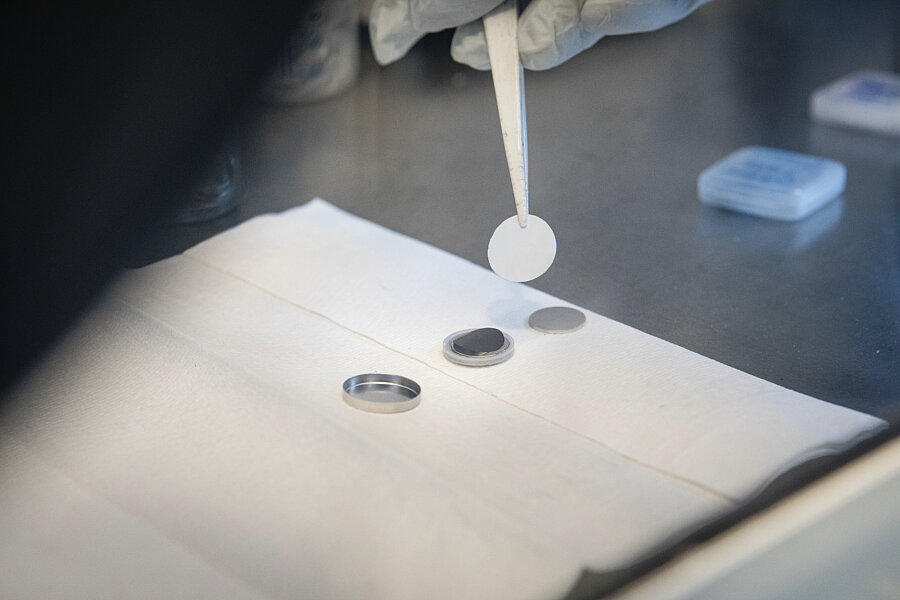
He did not take long to prove this. In this temple of tech, where he was given free rein to notably explore the properties of materials—with a view to further improving the effectiveness of lithium-ion batteries—he made a genuine breakthrough by successfully replacing the cobalt-based cathode with a manganese-based one. More virtuous than cobalt, whose extraction was an ethical and environmental catastrophe, manganese also offered better performance. Incidentally, it is this technology that the carmaker Renault used in its first ZOE cars.
The young researcher's career had barely begun, and he had already co-signed a half-dozen patents, while making his name in the community. Due to the antitrust legislation about to be passed in the United States, the Bell system had to be broken apart into a number of separate companies. His employers promised Tarascon that one of these new entities would hire him, but the contract never came. "Since I'm the type that takes my destiny into my own hands, I had job interviews with the competition, and quickly received an attractive offer from MIT. I immediately showed it to the management of Bellcore, and they signed my contract on the spot." It was out of the question to lose the French gem. In his new home—whose credo was "Do what you want, but be number one"—Tarascon set batteries aside for a while in favour of the fashionable topic of the time, one that he knew well from his postdoctoral fellowship, superconductivity.2 But an earthquake was about to make him quickly return to his favourite subject, and quite literally, as in 1989 a quake of 6.9 magnitude shook California. "The series of lead-based batteries intended to provide electricity for eight hours in the event of a blackout, which we had studied in an effort to improve them, gave out after just one hour," he recalls. "Bellcore was blamed for this error, and gave us a choice: either leave or get back to work on batteries, with the key being a major advance in the field if possible."
All they had to do was ask. In research and industry relating to lithium-ion batteries, Tarascon, who is now the father of a young boy, made a new breakthrough thanks to a "vision." "I always start by experimenting before I know how it will work, using intuition." Mischievous smiles light up his face during the interview, as he recounts his feats. "I believe that a researcher should not be limited to a bible of knowledge, but should instead question dogma, and strive to proceed differently than others."

What others were doing at the time were cylindrical batteries that were difficult to miniaturise and house in devices. To solve this problem, he came up with the idea of creating flat and flexible accumulators, and using a plastic case to hold the battery's active elements and the liquid electrolyte. "For such a ground-breaking idea to work, it must be shared with the largest number of people, in order to spark enthusiasm and attract talent. That is what I did during a coffee break at a seminar. I think I succeeded," he says, with a wry smile. "When he heard my idea, Paul Warren, who invented flexible wires for landline phones, and who was the head of a department at Bellcore, left his position to join my team." A wise choice. After four years of tests, the first flexible flat batteries were ready, and were presented to the public with great pomp during a press conference in 1994. It is thanks to this technology, which is protected by 25 patents, that today's lithium-ion batteries can power certain electric vehicles and smartphones.
Winning return to France
"In the United States, you never rest on your laurels. After this success, Bellcore of course asked me to stay on for a few more years, but with the commitment to come up with another striking discovery within four years. Such pressure is a powerful driver of innovation, but it is tiring over the long term." In 1995, he considered returning to France, but far from his native Aquitaine. The laboratoire des matériaux d’Amiens was looking for a new director, and to attract Tarascon immediately offered to make him an exceptional class professor, despite the fact that he had never taught. Moved by the generous proposal, he left America, the land of possibility, and crossed the Atlantic in the other direction. In Picardie he recruited a new team, revealing the temperament he developed during his fifteen years in the US. "He's an experimenter, someone who gets his hands dirty, even if it means sleeping at the laboratory to monitor a procedure," says Mathieu Morcrette, who worked with Tarascon for a long time, and now directs the laboratory in Picardie.3 Jumping ahead with Tarascon to a time when awareness was growing with regard to sustainable development, the group conducted pioneering research, especially on battery compounds produced from biomass, such as phytic acid, which is made from corn.
In France, Tarascon also showed his unifying spirit, desire to gather forces, and talent for advancing research. Upon his arrival, he implemented a prototype unit in which researchers from any laboratory could test the potential of their product without being dependent on companies. While he came up against the weight of the administrative system, and bore his share of criticism, he was nevertheless able to impose his idea, and soon created the Alistore European research network on lithium batteries, which is still active today. Driven by the same desire to bring people together, he then tried to create the French network for energy storage, but the burdensome administration he had to contend with was even more discouraging, such that he seriously considered returning to the United States, where all he had to do was pick up the phone to secure a prestigious position. "In 2010, UC Santa Barbara extended an offer that I accepted, with a start date of 1 April," he recalls. "On 29 March, one of my colleagues at the ministry4 learned that I was leaving, and immediately notified Valérie Pécresse, who was the minister for research at the time. The next day I received a letter of engagement from her promising to finance 40 postdoctoral fellowships to create the famous French network that I dreamed of. The following day the then president of the CNRS, Alain Fuchs, met with me in his office to ensure me that he supported my project 100%." Tarascon thus remained on this side of the Atlantic, and the RS2E network was born: today it includes seventeen university laboratories, and an equal number of industrial ones.
A commitment to greener batteries
During these years spent uniting his discipline, he never stopped conducting research or questioning paradigms. Shortly after his arrival at the Collège de France in 2014, he shined a light on a new mechanism, anionic redox. "This involves using the electrochemical activity of oxygen, and not just cobalt (or manganese), to release more electrons, and to hence produce more energy, which in theory enables doubling up battery performance! I think it's the discovery I am the most proud of."

With his colleagues he also created sodium-ion batteries, an element that is thousands of times more abundant on Earth than lithium. "Sodium-based batteries are the future!" The Chinese company CATL, a major global battery producer, has understood this, massively investing in this technology. In 2018, with his partners at RS2E, Tarascon created Tiamat, a company specialising in sodium-ion: "We cannot continue massive extraction of lithium and cobalt (used to manufacture lithium-ion batteries), which raises ethical and environmental problems. We must turn to more virtuous materials, such as manganese and sodium."
The other avenue pursued by Tarascon to reduce the negative impact of batteries is to extend their life span. "That's my hobbyhorse!" he says with a huge smile, his dark eyes twinkling beneath his thick brows. "It's a major consideration, as batteries will become increasingly indispensable to our daily lives. Yet as they are charged and emptied, they begin to wear out, and it is unfortunately impossible to analyse their weaknesses, for they are inaccessible black boxes." Strongly inspired by medicine, our medallist strove to design intelligent batteries with fibre optic sensors that can measure their properties in real time. Two floors above his office, three doctoral students from his team are "taking the pulse" of a battery, as when we monitor the vital signs of a human being. "With this technology, which still needs to mature, we hope initially to optimise battery life span, and later to double it. Eventually, we hope to make in situ repairs for the most minor scratch, in an effort to make them last a very long time, thereby preserving the planet's resources." For someone who comes from the land, that is quite consistent indeed.
_____________________________________________________________________
How does a battery work?
A battery, or an accumulator, is a device that can produce and store electricity thanks to chemistry. It consists of two electrodes with different charges—an anode and a cathode—both immersed in conductive solution, most often a liquid, known as an electrolyte. In modern batteries, the anode and cathode are stacked on top of each other like a thin-layered pastry rolled onto itself, and then immersed in an electrolyte. The principle of how they function depends on the electrochemical potential of the materials used, which is to say their propensity to lose electrons (in which case we say the material is oxidised), or to gain them (in which case we say it is reduced). Scientists thus speak of a material's oxidation-reduction (or redox) potential.
This is what happens when we use a battery: the anode loses electrons, which are guided via an electric circuit to the cathode, which captures them. When some of the anode's atoms lose an electron and become ions, its charge balance is broken. To restore it, positive ions are released, and move toward the cathode inside the battery through the electrolyte. When the cathode captures all positive ions, the battery is empty. When you charge it, the electrons and ions in the device move in the opposite direction. The greater the redox potential of the materials used in the battery, the more it can accumulate and store energy. Over time, the scientists have tested a number of materials in search of the best mass/performance ratio, with a view to constructing batteries that are both light and high-performance.
Lithium, which has a high tendency to release electrons, quickly emerged as a leading material for anodes. It was initially combined in a pure state with various metals for the cathode, and various mixed aqueous electrolytes, although safety and performance issues arose depending on the combination. One combination, lithium-ion batteries, revolutionised our lives by considerably increasing storage capacity and charging speed. In these accumulators, the anode is made of graphite, the cathode of cobalt oxide, while the electrolyte—which contains a lithium salt—is deprived of water (whose chemical reactions lead to the loss of energy). The development of this ground-breaking technology earned John B. Goodenough, Stanley Whittingham, and Akira Yoshino the 2019 Nobel Prize in Chemistry.
- 1. AT&T Bell Laboratories, better known by the name Bell Labs.
- 2. A material cooled to a very low temperature acquires the capacity to perfectly conduct an electrical current with no resistance, and hence no loss of energy, is said to be superconducting.
- 3. Today it is now known as the Solid-State Chemistry and Energy Laboratory (CNRS/Université de Picardie Jules Verne).
- 4. Ronan Stephan.