You are here
Soft Chemistry: Naturally Creative

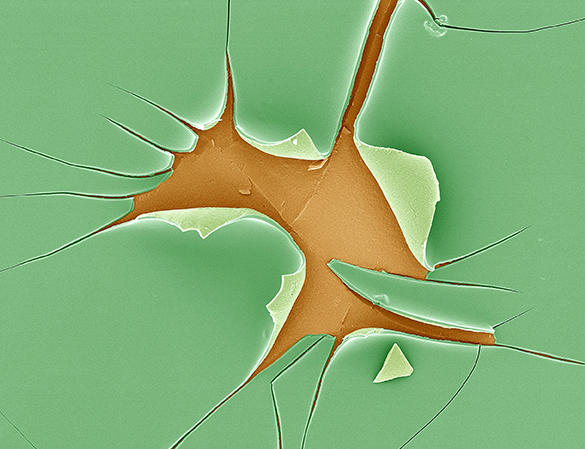
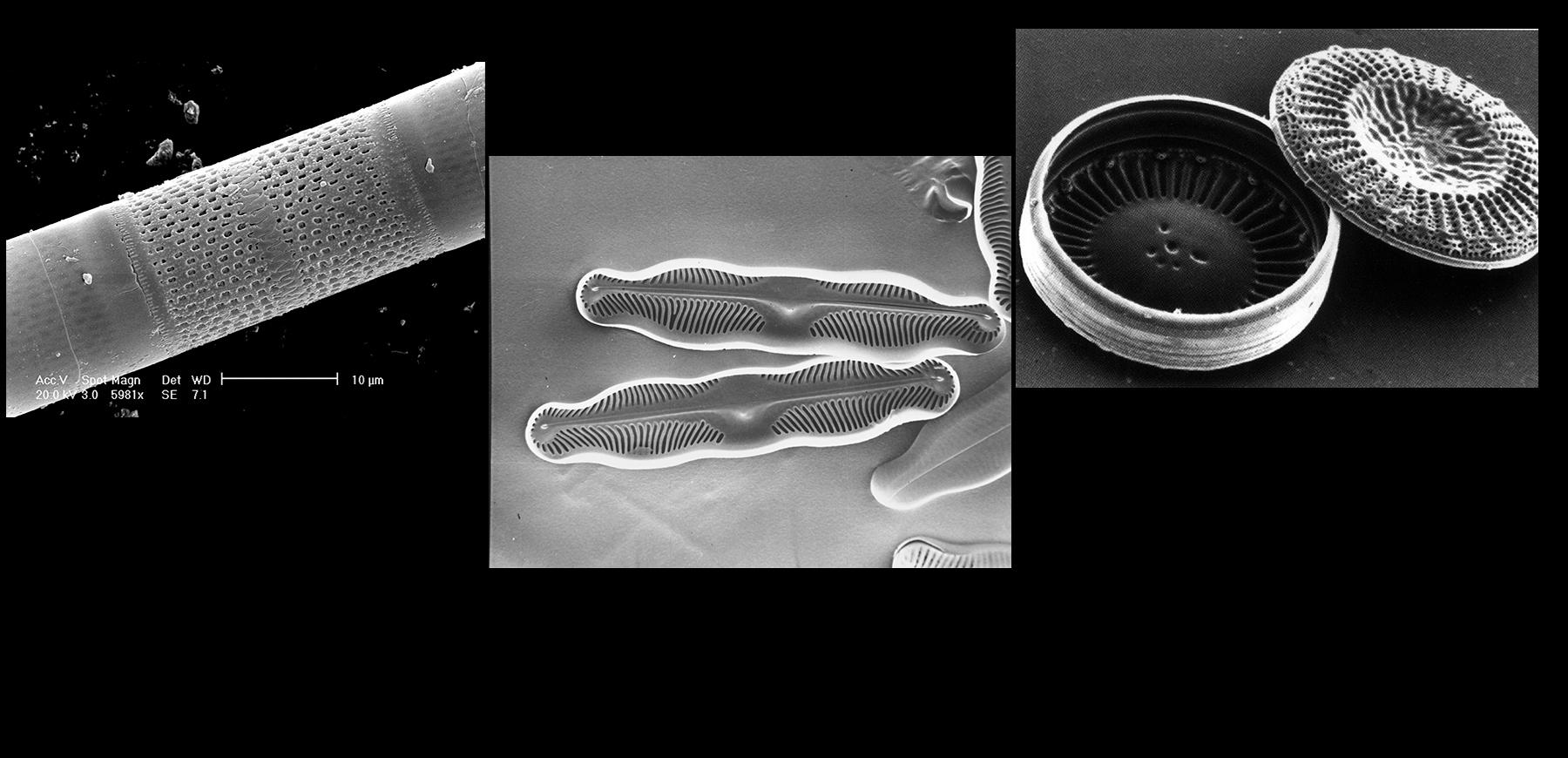
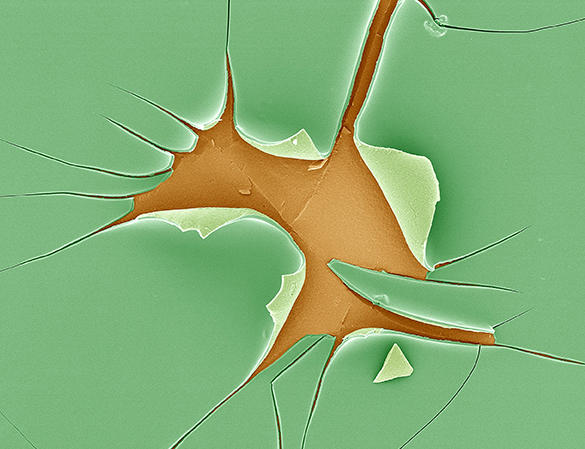
Diatoms are fascinating organisms. Found in abundance in lakes and rivers, these unicellular microscopic algae, whose structure fascinated Charles Darwin, are able to produce a glass shell using silica dissolved in water. Unlike our glassmakers, they do so at room temperature.
The emergence of sol-gel processes
This example provided great inspiration for Jacques Livage, a member of the Académie des sciences. Beginning in the late 1970s, he became one of the primary contributors to the scientific development of sol-gel processes (contraction of the terms solution-gelation), whose basic principle is not different from that of the diatom. Take some silica—the main component in sand—dissolve it in water, and proceed with a simple polymerization process in the presence of catalysts, while adjusting the pH. You will obtain glass in much milder conditions than those traditionally used by industry, which consist in melting sand by heating it to 1500°C. "Although patented in 1939 by the Schott Company, this process was curiously ignored by the academic world until the 1980s!" stresses Livage.
This was the dawn of soft chemistry. The term was invented in 1977 by Livage himself (in French: chimie douce), who has since then advocated a chemistry "that harmoniously fits into natural processes." More precisely, the researcher defines it as "a series of methods for producing inorganic or hybrid materials involving polycondensation reactions at low temperatures—from 20°C to 200°C—all of which starting from particles suspended in an aqueous or organic environment."
Hybrid materials made to measure
Not only is the advantage of working at low temperatures obvious in terms of energy saving, but the process also provides great control over the structure that is being created, since the suspended particles aggregate gradually. To put it plainly, it makes it possible to produce materials that are tailor-made for the desired application. "Sol-gel processes do not yield much solid product, as they involve working in a solution, and the complete evaporation of water leaves behind a powder," Livage points out. "On the other hand, they are very well-suited for depositing thin films."
Applications have mushroomed since the 1980s. For example, glassmakers have developed this process to deposit anti-reflective coatings on windows or windshields. High technology sectors such as the space industry are no exception, notably with the refractory tiles of the Space Shuttle Columbia, which were produced using ceramic fibers obtained through a sol-gel process. Better still, researchers have expanded the possibilities of operating at low temperature and with high precision by developing novel, hybrid organo-mineral materials.
Inconceivable under traditional chemical approaches, they combine both mineral and organic components. For example, films made of titanium dioxide (Ti02) have made it possible to develop self-cleaning glass, such as that used on the rooftop of National Grand Theater of China in Beijing. The titanium dioxide particles have the property of decomposing organic particles (such as dirt) through photocatalysis, while the hydrophobic microstructure repels water. A perfect example of a tailor-made material. Today, hybrid materials are everywhere, from the soleplates of irons to fabrics, cars and optics.
Encapsulating bacteria for better use
In addition to past applications, the discipline continues to develop materials able to address the needs of the future, in particular bio- and "living" materials. "Just as diatoms cover themselves in glass, we can now encapsulate living microorganisms," Livage explains. Numerous microorganisms such as bacteria, fungi, micro-algae, plant and even animal cells have thus been immobilized within silica gels, while preserving their biological activity.
By finely controlling the porosity of the capsule, scientists are able to maintain the microorganism's exchanges with the external environment. This could result in applications ranging from medicine to the environment. Thanks to this process, bacteria that are sensitive to certain chemical species serve as anti-pollution sensors or immunity tests for medical analysis, while others are used for their ability to eliminate certain substances or to produce medical compounds. Teams of researchers are developing sol-gel reactors that fix these microorganisms in order to increase their productivity.
In medicine, encapsulation also helps to protect certain cells. For example, scientists have succeeded in injecting diabetics with the pancreatic cells involved in the production of insulin. The shell protects these foreign cells from the patient's immune system. Nanomedicines also represent a promising avenue, as they are small enough to penetrate diseased cells, especially cancerous ones, and destroy them from the inside. Using encapsulation, researchers have equipped these nanomedicines with stealth and targeting functions that will enable them to reach the relevant cells more directly, which could lead to more targeted and efficient therapies.
Producing nanomaterials
Another great prospect for soft chemistry is nanomaterials, a field in which researchers have drawn inspiration from sponges. Some are developing silica fibers, called spicules, which can settle on the sea floor. Franck Artzner, a researcher at the Institute of Physics in Rennes,1 and his team have attempted to reproduce the protein at the heart of these spicules, which can accumulate successive layers of silica suspended in water, thereby obtaining silica nanofibers that can self-assemble. By modifying the properties of this protein, other teams have succeeded in fixing films of cobalt oxide on these nanofilaments, thus creating electrodes that can double the energy capacity of a lithium battery!
Sponges, algae... our environment is an inexhaustible source of ideas. "Soft chemistry is first and foremost a bio-inspired chemistry," Livage points out. This biomimetic approach, based on the observation of hitherto unsuspected natural processes, moreover connects with green chemistry, another promising field. Both shape the chemistry of the future, one that respects the environment and provides the materials of tomorrow. A sign of the times, in March 2015, Ségolène Royal, France's Minister of the Environment, Energy and Marine Affairs, inaugurated the Centre européen d’excellence en biomimétisme de Senlis (CEEBIOS). Soft chemistry holds a few surprises in store!
- 1. CNRS / Université de Rennes 1.